Introduction
While Felix d’Hérelle was studying an outbreak of dysentery among soldiers in Paris in 1915, he discovered that a microbe was lysing and clearing bacteria.1 The microbe was soon characterized as a bacteriophage, and d’Herelle began experimenting with phage to fight bacterial infections. D’Hérelle worked closely with George Eliava from Georgia, who studied phage treatments with d’Hérelle at the Pasteur Institute in Paris from 1919-1921, 1925-27, and 1930-31 against Vibrio cholera, Escherichia coli, Shigella flexneri, and Salmonella typhi.2 In 1923, Eliava established the Eliava Institute of Bacteriophage, Microbiology and Virology in Tbilisi, Georgia. The Institute survived the rise and fall of the Soviet Union and was owned by the Soviet Ministry of Health for a while during its peak period. Today, it includes multiple companies that produce commercial phage preparations and an outpatient day clinic which treats hundreds of domestic and international patients each year with phage therapy.2 Russians were particularly interested in phage as it fit within their plan to mobilize science for the new socialist state plagued by outbreaks of cholera and dysentery.1 Phage therapy was a safe treatment with very few adverse effects, but it was mostly developed within France, Russia, and Georgia. A string of critical reviews on phage therapy in JAMA in the 1930s likely contributed to the U.S.’ resistance to phage therapy.3
During and after World War II, antibiotics were the preferred antimicrobial. Antibiotics were more convenient because they cleared a broad spectrum of bacterial strains quickly and consistently. Since antibiotics could be used on a broad spectrum of bacteria, it did not require laboratory tests identifying the bacteria to prescribe. However, antibiotics can reduce healthy microbiota diversity, which can compromise the immune system.4
The growing risk of antibiotic resistance is prompting an international global health response. Resistance emerged shortly after antibiotics were introduced in hospitals; sulfonamide-resistant Streptococcus pyogenes emerged as early as the 1930s, penicillin-resistant Staphylococcus aureus emerged in 1942, and streptomycin-resistant Mycobacterium tuberculosis emerged in 1946, just 3 years after streptomycin was first used.5 Widespread multidrug resistance emerged in the 1950s and 1960s and accelerated as antibiotics became a standard treatment.6 By 2001, the World Health Organization developed a global strategy report for containment of antimicrobial resistance, targeting patients, prescribers, hospitals, agriculture industries, national governments, and health systems.7 Today, the Centers for Disease Control and Prevention estimates that nearly 2.8 million antimicrobial-resistant infections occur in the US every year, contributing to a growing total of 48,000 deaths and an annual cost of $55 billion.8
Basic research in network biology and gene regulatory networks may lead to advances in understanding resistance.9 Robust laboratory research has continued in the US to understand microbial processes, but phage therapy is still limited to compassionate therapy. Compassionate therapy refers to using a therapy that has not been approved for the purpose of treating a serious condition and all other treatment options have been exhausted. The relative success of antibiotics has contributed to a lack of understanding and documentation of phage in vivo. Leading US laboratories focused on antibiotic-resistant research are now revisiting phage therapies as solutions to combat emerging antibiotic-resistant strains.10
Phage and Phage Therapy
Phage Characteristics
Phages are viruses made of densely packed immunogenic DNA or RNA encapsulated in a protein coat.11 The approximately 1029 to 1031 phages in the biosphere naturally modulate bacteria by bursting bacterial cells (lytic phage) or inserting their genetic materials into bacteria to integrate with the bacterial genome (lysogenic phage). Because phage and bacteria evolve together to ensure each other’s survival, bacteriophages in their natural environment are typically less virulent than synthetic bacteriophage. In general, lytic phage are better for phage therapy. Lytic phages lyse specific bacteria by inserting their DNA into the bacterial genome and using the cellular structures and functions to replicate its DNA and reassemble multiple copies of itself inside the bacterium before lysing (bursting) the cell. Lytic phage is the preferred type for phage therapy.
Phage Therapy: Process for Identification and Selection
Researchers often look for phage in the same place bacteria grow. This is why hospital sewage is the most common place to collect phage strains for therapy.12 Once phage is identified and characterized, the phage can be catalogued into a phage bank. Then, phage must be grown (enriched) in bacteria and purified and filtered into a phage stock. Once the phage is identified and catalogued, physicians need to obtain the phage for therapy. In early history, phage preparations were simple suspensions of phages in liquid that were taken orally or administered intravenously.13 Natural phage inherently has a low toxicity.10 Phage therapy uses repeated administration of phages over time to clear the bacterial infection orally, topically, or intravenously.
More than 5000 individual phages have been isolated from the environment, and 750 phages have been completely sequenced.14 A total of 70% of characterized phages infect only 12 bacterial hosts The diversity of uncharacterized phage is encouraging for the future of phage therapy. If a bacterial strain develops resistance to phage, there is likely another phage strain with the potential to target it. This is encouraging in the context of widespread antibiotic resistance and quickly advancing gene sequencing technology.
Phages have been identified and tested as treatments against all bacteria in the World Health Organization’s critical list, including carbapenem-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae, as well as against bacteria of high priority including Enterococcus faecalis, S aureus, and Salmonellla spp, which cause severe infection and show high resistance to fluoroquinolone antibiotics.15 The most common bacteria targeted in current clinical trials are P aeruginosa, S aureus, Escherichia coli, and Enterococcus. Clinical trials are conducted exclusively at hospitals affiliated with universities in the US and Europe, with a few outlying hotspots in Israel (Figure 1). Phage therapy products come in the form of topical solutions, intravenous infusions, inhalation, and dietary supplements for clinical indications, ranging from a compromised gut microbiome from Crohn disease, urinary tract infections, diabetic foot, and chronic pulmonary infections in patients with cystic fibrosis. For example, the WRAIR-PAM-CF1 bacteriophage product produced by the National Institute of Allergy and Infectious Diseases is a 4-phage cocktail administered intravenously that targets P aeruginosa in patients with cystic fibrosis. The first patient was treated in early 2023 by Adaptive Phage Therapeutics to test the safety of the product.16
Phage Therapy Risks
The goal of phage therapy is not to replace antibiotics, but to diversify physicians’ repertoire and knowledge about treatment options for bacterial infections to avoid overprescribing any one product. The risks of phage therapy are highest if physicians begin prescribing 1 or a few synthetic phage products for even minor infections, giving bacteria more exposure to 1 phage and opportunities to develop resistant mutations.
In 2002, University of California San Diego (UCSD) professor Lin Chao found that RNA bacteriophage φ6 has a low replication rate, allowing it to avoid the fitness costs of a high deleterious mutation rate.17 His years of studying viral and bacterial replication have formed his opinion that phage resistance is a risk, and that one way to slow resistance to a manageable level is to use antibiotics and phage in combination.18 Targeting multiple bacterial functions could lead to mechanistic trade-offs that reduce the fitness of resistant phenotypes. Resistance is costly to bacteria. A study of 17 unique resistance mutations found that most mutations converged in 1 of 4 genes, all involved in synthesizing 3 outer membrane lipopolysaccharide molecules and that all resistant mutants grow more slowly than wild-type strains.19 Phage-resistant strains of P aeruginosa showed fitness costs that affected their mobility, ability to form biofilm, and increased their susceptibility to antibiotics.20 However, some bacteria can regain their fitness by acquiring compensatory mutations and can acquire these mutations in parallel across lineages, rather than through inheritance.21 In summary, bacteria evolve and retain their resistance to phages similarly to antibiotics.
One outcome of phage resistance is increased antibiotic susceptibility when a small (sublethal) dose of antibiotics is combined with phage treatment.17 Sublethal doses often produce filamentous strands of bacteria that are more vulnerable to phages. Also, there is substantial evidence that sublethal doses of antibiotics can stimulate cells to produce more phages to clear infection more quickly, also known as phage-antibiotic synergy. Because bacteriophages are viruses and replicate naturally in the human body, phages can evolve to resist bacterial resistance. For example, restriction-modification systems in bacteria recognize and degrade phage DNA,22 but phage can encode antirestriction and antimethylation proteins ArdA, KlcA, and their homologues, as shown on carbapenemase-resistant Klebsiella pneumoniae.23 In addition, phages use host bacteria CRISPR/Cas systems to integrate their DNA into short pieces of RNA that direct the degradation of phages.24 In turn, phages can use their own CRISPR/Cas systems to degrade bacterial DNA. The ability for bacteriophages to evolve with bacteria makes them a promising approach to evade resistance. Knowledge of the natural mechanisms of phages to counter resistance are crucial in developing synthetic phages by using CRISPR/Cas systems. Synthetic phage can be targeted at a more specific or broader range of bacteria, and target genes involved in resistance.
Extending the Potential of Phage Therapy: Phage Engineering
The same mechanisms of resistance that bacteria can use to fight antibiotics can be used to develop phage resistance. Bacteriophages are viruses with the ability to replicate inside the organism and evolve resistance. The dynamic between bacteriophage viruses and bacteria is complex and often referred to as an “arms race” as the two evolve to outcompete the other’s resistance mechanisms.25
New methods in genetic engineering are making it possible to engineer a more effective phage product. The first successful treatment using engineered phages was in 2019 in a patient with cystic fibrosis infected with antibiotic-resistant Mycobacterium abscessus.26 This case used the bacteriophage recombineering and electroporated DNA (BRED) technique to engineer 2 phages as part of a 3-phage cocktail. BRED is a promising modern technology that uses the machinery of bacteriophage to recombine homologous DNA sequences in vivo.27 The goal of this technique is to increase the percentage of recombined phages in bacteria. BRED is more efficient at engineering viral genomes than traditional recombination methods, especially for bacteria such as Yersinia pseudotuberculosis, Shigella, Pantoea ananatis, P aeruginosa, and Salmonella enterica. However, it is inefficient compared with newer methods incorporating CRISPR/Cas systems into phage products.28
CRISPR/Cas9 systems make phage products more effective at targeting bacteria. Recombinant bacteriophages can be engineered to carry and deliver CRISPR/Cas systems that kill bacteria more efficiently. CRISPR/Cas systems include a Cas9 protein and guide RNAs that target virulence genes in bacteria and can be inserted into bacteria through the phage capsid.29 Phage products that deliver CRISPR/Cas systems are significantly more effective than BRED techniques alone.28 These CRISPR systems have become an invaluable tool in synthetic biology to delete and replace genes to target specific cellular responses.29
These methods are still in their beginning stages but using CRISPR/Cas9 systems in phage engineering is crucial to improve the efficacy and consistency of phage therapy products against resistant bacteria.
International Phage Therapy
The number of publications on phage therapy has been exponentially increasing since 2001.30 This analysis shows that the U.S. and China generate the most publications on phage therapy in bacterial infection and collectively produce more than twice as much research on this topic as all other countries combined. However, research on bacteriophages indicates a broad range of applications beyond the clinical setting, including animal, food, and plant uses.31 Bacteriophages can also be used as a research tool to detect the presence of certain bacteria, which is useful in food science and agriculture.
Soviet Union
During the Russian Revolution of 1917, Soviet leadership prioritized fighting infectious disease as part of their plan to construct a healthy nation. In 1918, the Commissariat of Public Health was established, marking the beginning of state-owned health care focused on prophylaxis (disease prevention) through propaganda.32 The Commissariat established a network of the Institutes of Microbiology and Epidemiology in major cities to serve the regime. In addition, the obstacle of patenting was not an issue in Soviet Russia as it was in the west. All these conditions made it possible for phage therapy to proliferate in the USSR. Based on the findings of 17 clinical trials conducted between 1921 and 1940, phage therapy was effective, but less effective than standard treatments.33 Phage therapy was most effective as treatment for dysentery and prophylaxis for potential outbreaks of cholera in cities. The Soviet Union continued to use phage therapy through World War II and the Cold War while many other countries favored antibiotics because it was more profitable for pharmaceutical companies.
The Eliava Phage Therapy Center (EPTC) receives custom phage orders from all over the world in addition to providing for their patients in the clinic.34 At a phage conference in 2019, a physician at the EPTC reported that the center treated 400 patients with phages in 2018 and 20 to 30 patients with cystic fibrosis per month.35 In addition, they reported that they “has been trying for a decade now to get through the bureaucratic procedures of the United States and of the European Union regarding bacteriophage therapy.”
The phage therapy unit at the Hirszfeld Institute of Immunology and Experimental Therapy in Poland was created in 2005 as the first ethically approved phage treatment facility in the European Union, which is important because Polish law has not authorized phage therapy as a marketable treatment.36 Instead, it is an experimental treatment regulated in accordance with the World Medical Association’s Declaration of Helsinki, which determines guidelines for compassionate care. Like Georgia, Poland continued to use phage therapy through political turmoil and the dissolution of the Soviet Union. The Hirszfeld Institute treats patients with phage preparations. It reported treating 1307 patients from 1987 to 1999, conducting 3000 phage-typing procedures, and successfully identifying active phages in an annual average of 80% of cases.37 A 2001 report from the institute demonstrated that 85.9% of patients who received intravenous phages three times a day experienced full recovery from infections primarily caused by Escherichia, Klebsiella, Proteus, Enterobacter, Pseudomonas spp, or S aureus bacterial strains.38 The institute noted a decreasing number of registered patients, attributing this decline to more phage therapy centers opening around the globe. In addition, the phage therapy unit has not yet conducted clinical trials.
Israel
In 2018, the Israeli Phage Therapy Center (IPTC) was established in response to the growing threat of antibiotic resistance. Since then, the center has received 196 requests from hospitals that send their patients’ bacterial isolates (half of which were antimicrobial resistant) for testing against potential phage therapeutics. The IPTC characterizes phage isolates, tests for bacterial susceptibility to phages, authorizes treatment, and delivers and supervises phage therapy. The center has treated 20 patients in the last 5 years, primarily for bone infections.39 The IPTC tests bacterial isolates for phage susceptibility, performs an antibiotic checkerboard assay, collaborates with researchers to identify and purify associated phages, receives approval from the Institutional Review Board and Ministry of Health, and administers the phages. The IPTC uses a program called REDCap to store all the data in the same place. The treatment cycles last 6 to 45 days and the follow-up is 1 to 3 years. The center has reported a success rate of 78%.37 In May 2023, the IPTC entered an agreement with Adaptive Phage Therapeutics in Maryland, US, to allow the company to evaluate phages identified by the IPTC.
Phage Therapy in the US
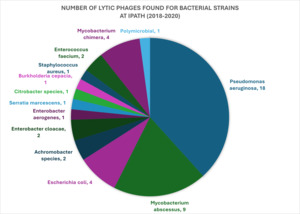
Although phage therapy has been approved by the US Department of Agriculture/Food and Drug Administration (FDA) for use in animals and plants, the FDA has not yet approved any bacteriophage products for human clinical use.40 This means it is not covered by insurance. The approval process for phage products to treat a chronic or life-threatening bacterial infection in the US requires an application for single-use Investigational New Drugs (IND) to use phage as therapy. At the Center for Innovative Phage Applications and Therapeutics (IPATH) at UCSD, it takes a median of 171 days to administer phage treatment from the time of the provider’s request.41 IPATH also reports that phage therapy was both safe and effective in clearing 7 of the first 10 infections that IPATH played a role in treating since its founding in 2018. Most of the lytic phages they have found (18 of 47) target Pseudomonas aeruginosa, for which they have received 92 requests.40
Physicians can submit requests to use phage for compassionate care when the patient’s case meets a list of criteria. The FDA approves phage therapy for a patient only when:
-
A patient cannot enroll in a clinical trial; the infection is severe or life-threatening.
-
There is no comparable therapy available.
-
The patient justifies the risk of treatment.
-
Approving the medical product in their case won’t interfere with ongoing investigational trials.42
In the US, the National Institute of Allergy and Infectious Diseases, Adaptive Phage Therapeutics, Intralytix, Locus Biosciences, UCSD, and Armata Pharmaceuticals are currently conducting clinical trials.43 Most clinical trials are multicenter, double-blind or triple-blind, and administer a phage cocktail and a placebo every day for several weeks.
Phage specificity and their variable behavior in different microbiomes make it challenging to coordinate large-scale clinical trials. A single phage strain must pass a standard of potency by clearing bacterial infections in potentially thousands of individual hosts. However, bacteriophage treatment is highly individualized to the patient. For example, the susceptibility of M abscessus infections to phage varies considerably from patient to patient.44 Some isolates are efficiently lysed by 1 phage and others show resistance based on their bacterial morphotype. In addition, the variability of phage replication in humans after administration makes it difficult to set a standard dose.45 This is important because the FDA regulates biological products based on dosage. The “virulence index” is a way to measure virulence using a multiplicity of infection, or the ratio of phage to bacteria. The index quantifies virulence as affected by environmental and physiological factors such as burst size, adsorption rate, latent period, and phage therapy efficiency.46 Quantifying virulence may help researchers predict how phages will replicate in humans after administration and serve as a regulatory standard. In addition, simple phage isolates, such as the ones available over the counter in Eastern Europe, are considered natural products that can’t be patented.47,48 To profit from phage therapy, producers must genetically engineer them or patent the engineering process.
Recently, the FDA has been accepting more innovative clinical trial designs because of the difficulty of obtaining sufficient benchmarks or large enough clinical trials in personalized medicine through traditional clinical trial designs.49 In response to the change in clinical trial designs, the FDA published a guide for industry trials for nontraditional biologics.49 The report established that the trial results must still be able to predict the actual effects of treatment and avoid bias at all costs. Modifying the sample size should only happen based on a prespecified formula (rather than modified after the trial has begun or based on a hypothesis) and include blinded or masked analyses during the trial to avoid changing the course of the trial based on interim data. They use the term adaptive enrichment design to describe a trial that defines a different standard of success for a subset of the sample population but includes the complementary subpopulation. Another type of adaptive enrichment design is a Bayesian random partition design. In this design, patients are assigned a subgroup-specified treatment based on their biomarkers.50 These designs are overseen by the Center for Drug Evaluation and Research’s Antibacterial Drug Development Task Force, which includes members from diverse FDA offices.51 Overall, adaptive trial designs are still subjected to the same rigorous review as traditional trials, but sample sizes, biomarkers, end points, and how researchers decide to partition treatment groups vary. Therefore, it is still more difficult to get phage products to market in comparison with traditional antibiotics because phage therapy is more individualized to the patient.
Besides research institutions, many federal agencies are partnering with the private sector to develop antibiotic alternatives. For example, the Biomedical Advanced Research and Development Authority partners with Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X).52 CARB-X’s first bacteriophage program to enter clinical trials is SNIPR Biome, which completed a phase 1 trial in 2022. SNIPR Biome’s cocktail of 4 CRISPR-engineered bacteriophages, named SNIPR001, targets E coli fluoroquinolone-resistant strains in biofilms. Other phages being used to target E coli in clinical trials are EcoActive, LBP-EC01 (for urinary tract infections), WPP201, and PYO phage (Table 1).
Conclusions
The rising cost of antibiotic resistance is pushing the US to reconsider alternative treatments such as phage therapy. While phage therapy is an established treatment in Eastern Europe and Israel, alternative treatments for bacterial infections with phage are still rare in the US. Evidence for the success of phages in eradicating infection is abundant in isolated international cases. Successful cases must be accumulated to use phage therapy widely in the US. The limiting factors of phage therapy in the US might be the following: (1) its association with Russia and the Soviet Union during the Cold War, (2) the established market for antibiotics, (3) incompatible regulatory standards for alternatives, (4) difficulties of personalized treatments in the past, and (5) limited understanding of phage DNA and its interactions with bacteria in vivo. At the IPATH laboratory, patients experience the same benefits that once defined the beginning of the antibiotic revolution. Patients at IPATH have reported that phage therapy is cost-effective, reduces the risk of acquiring hospital infections, and allows them to continue their routines uninterrupted. Further clinical trial evidence of phage therapy efficacy in vivo and research on phage resistance mechanisms will guide decisions on which phage to develop for mass distribution. More effective phage products driven by the combination of clinical documentation and basic research will improve the outlook for phage therapy as a tool to combat antimicrobial resistance.