Introduction
Acute rhinosinusitis (ARS) is a common and costly condition.1 Sinusitis accounts for “expenditures of approximately $3.5 billion per year in the United States.”2 Rhinosinusitis is “symptomatic inflammation of the paranasal sinuses and nasal cavity.”3 The etiology of ARS is most commonly a viral infection.1 Although rhinovirus, influenza, and adenovirus are some of the more common viral pathogens responsible for ARS, ARS can also be a bacterial infection.1 Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are some of the more likely bacterial species to cause ARS.4
Although most ARS is caused by viral infections, antibiotics are oftentimes prescribed to treat ARS.1 A study found that sinusitis was the diagnosis most associated with antibiotic prescription.5 The same study also found that in the US in 2010-2011, there was an “annual antibiotic prescription rate per 1000 population of 506, but only an estimated 353 antibiotic prescriptions were likely appropriate.”5 The overuse of antibiotics has led to numerous organisms that have become resistant to antibiotics, making these previously effective medications less useful to treat bacterial infection.6 However, given that ARS is mainly a clinical diagnosis, it can be quite difficult for clinicians to distinguish whether a case of ARS is viral or bacterial in nature.1
Clinical guidelines recommend that ARS symptoms lasting 10 days or longer are more indicative of a bacterial infection.4 If there was a way to identify who would benefit from antibiotics earlier, it could save morbidity and money. Some previous studies suggested clinical findings that were indicative of a bacterial infection in the case of ARS.7–10 Some potential indications for the presence of ARS include C-reactive protein (CRP) level, purulent nasal discharge, and physician impression/gestalt. CRP levels have typically been used to measure inflammation. It was found that CRP levels greater than 10 mg/L had an overall positive likelihood ratio of 1.84 of diagnosing bacterially caused ARS.7 Another study found that purulent nasal discharge had a positive likelihood ratio of 1.6 (95% CI, 1.4-1.7) when it came to diagnosing bacterially caused ARS by any reference standard.8 Further, physician impression/gestalt has been described as an approach to diagnostic decision-making that is not reliant on algorithms or analytics, but rather on impression and intuition.9 One meta-analysis found that across multiple studies, the positive likelihood ratio of diagnosing bacterial ARS based on clinical gestalt was 3.9 (95% CI, 2.4-5.9).9
We conducted a randomized, pilot, 4-group study called the Nasal Steroids, Nasal Irrigation, Oral Antibiotics, and Subgroups Targeting for Effective Management of Sinusitis (NOSES) trial. There were 3 main aims of this study. First, to compare, through patient-reported outcomes, the efficacy of oral antibiotics (amoxicillin-clavulanate) and intranasal corticosteroids (INCS) for clinical improvement of ARS among patients who do not improve with supportive care alone. Second, to identify which patient subgroups benefit most from oral antibiotics (amoxicillin-clavulanate) vs INCS. Third, to identify which patient subgroups improve with supportive care and do not require antibiotics or INCS. This article focuses on a substudy of the Patient-Centered Outcomes Research Institute (PCORI) NOSES study and reports on the second aim, identifying subgroups that may benefit earlier from antibiotic interventions in the case of ARS.
Methods
Data for this study were collected from participants enrolled in the pilot PCORI-funded NOSES study. Participants ranged from 18 to 65 years of age. Participants presented to their clinicians with symptoms consistent with ARS. After meeting both the inclusion and exclusion criteria, participants were enrolled in the NOSES study. After enrollment, participants and their clinicians filled out a baseline survey containing clinical information and context about the participants’ ARS symptoms. This substudy compared some of these baseline data between participants with resolved vs prolonged ARS symptoms.
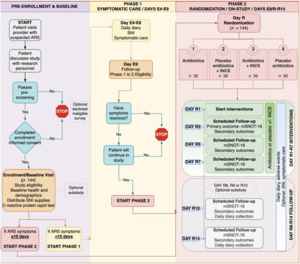
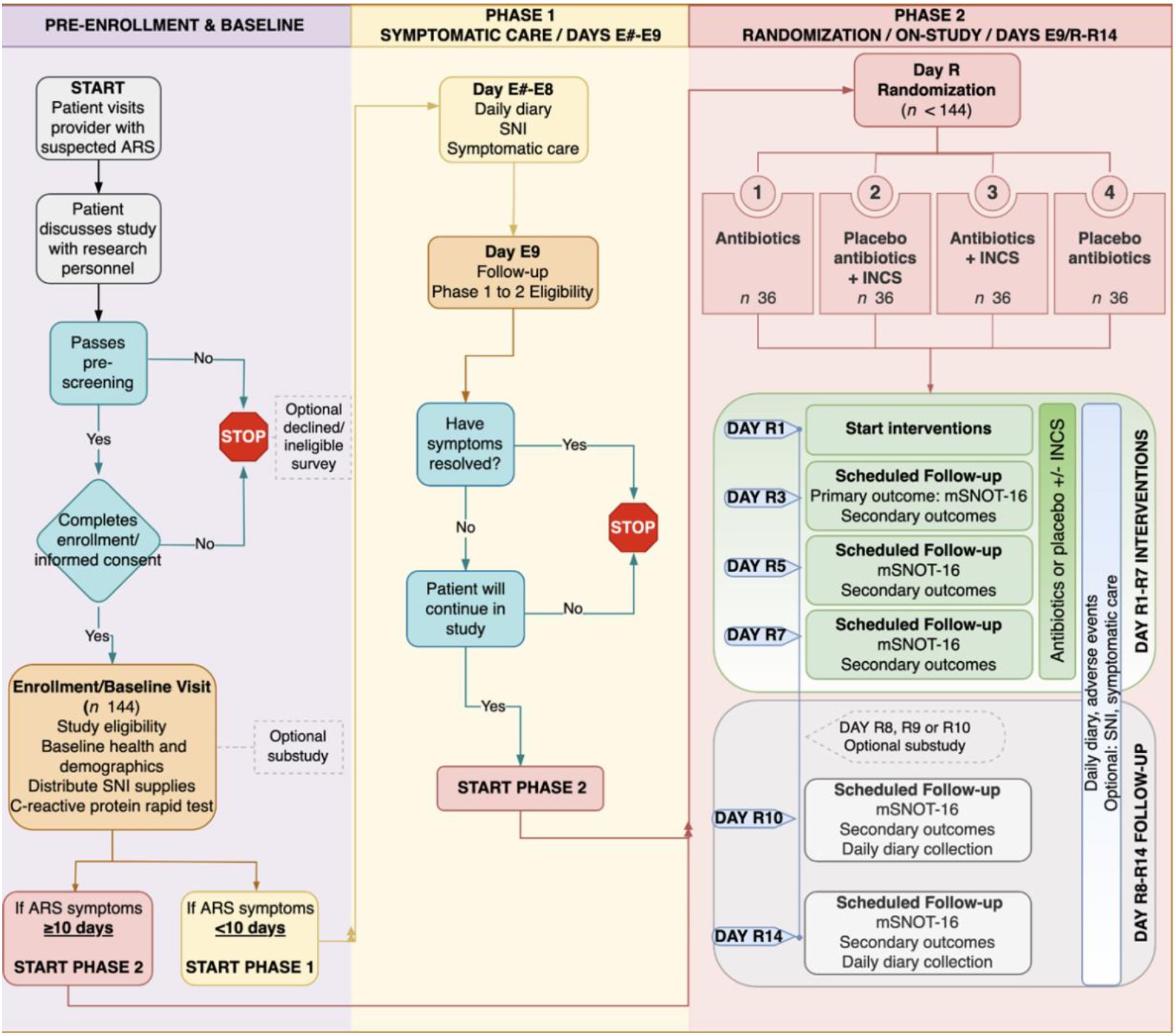
After the preenrollment and baseline phases, participants were categorized into 2 further phases (Figure 1). Participants placed in phase 1, defined as the symptomatic care phase, presented with ARS symptoms for more than 10 days. During the symptomatic care phase, participants were given clinical over-the-counter suggestions (acetaminophen, guaifenesin, dextromethorphan hydrobromide, and pseudoephedrine) and saline nasal irrigation. Within the symptomatic care phase, there were 3 types of participants: (1) participants with resolved ARS symptoms that improved and did not need to transition to the randomized clinical trial (RCT) phase; (2) those with ARS symptoms who did not improve, but received antibiotic therapy by their clinician during the 9-day symptomatic care phase and did not progress to the RCT phase; and (3) those with ARS symptoms that did not improve during the 9-day symptomatic care phase and progressed to the RCT phase.
Phase 2, which is the previously mentioned RCT phase, consisted of 2 groups of participants with prolonged ARS symptoms. The first being those from the symptomatic care phase whose ARS symptoms lasted longer than 9 days. The second being those who presented at baseline with ARS symptoms already lasting 10 days or longer. This group bypassed the symptomatic care phase and automatically progressed to the RCT phase. Participants from these 2 groups were randomized into 1 of 4 treatment groups. Group 1 received antibiotics, group 2 received placebo antibiotics plus INCS, group 3 received antibiotics plus INCS, and group 4 received placebo antibiotics. These groups were then followed up for 14 days during which the severity of ARS symptoms was assessed using a scoring system called modified Sinonasal Outcome Test-16.
These baseline data were presented in 6 subgroups, with 3 of them having a positive indication and 3 having a negative indication. A positive indication meant a laboratory value met a certain threshold, the clinician had an impression that there was a high or intermediate probability that the diagnosis was bacterial in nature, or there was the presence of a certain clinical finding. A negative indication meant a laboratory value did not meet a certain threshold, the clinician had an impression that there was a low probability that the diagnosis was bacterial in nature, or a certain clinical finding was absent. CRP, clinician gestalt/impression, and purulent nasal discharge are the factors that determined the positive or negative baseline subgroups. Positive indications included CRP levels greater than or equal to 10 mg/L (CRP), a high or intermediate probability that the patient had bacterial sinusitis according to clinical impression (clinician gestalt), or the presence of yellow to green and thick nasal discharge (purulent discharge). Negative indications included CRP levels less 10 mg/L (CRP), a low probability that the patient had bacterial sinusitis according to clinical impression (clinician gestalt), or the absence of yellow to green and thick nasal discharge (purulent discharge).
The percentage of participants with prolonged ARS vs resolved ARS symptoms was measured in each of the 6 baseline subgroups. These percentages among each subgroup were compared to see which subgroup had the highest percentage of both participants with prolonged ARS and those with resolved ARS. Likelihood ratios between what was observed in this study vs those in previous literature were also compared.
Additionally, among the participants who had prolonged ARS symptoms, the number of positive baseline subgroups they were each individually a part of was assigned. The positive baseline subgroups included a positive CRP level of 10 mg/L or greater, a positive clinician gestalt/impression that the participant had bacterial sinusitis, and a positive presence of purulent nasal discharge. These participants could either be assigned to 3, 2, 1, or zero out of 4 of the positive subgroups. The relationship between the number of positive subgroups a participant was a part of and the percentage of participants with that number of positive subgroups having prolonged ARS symptoms was observed.
Results
There was a total of 140 participants enrolled in the pilot study. Among these participants, 96 had prolonged ARS symptoms and 44 had ARS symptoms resolved in less than 10 days. Table 1 presents the demographic data from the participants. Table 2 presents the 6 subgroups previously mentioned. Within each subgroup, the percentages of participants with prolonged ARS vs resolved ARS symptoms are presented. It should be noted that some participants did not report all of their baseline data or data were not collected. Table 3 presents the observed positive and negative likelihood ratios compared with previous literature. Figure 1 depicts the relationship between the number of participants that were included in the 3, 2, 1, and 0 positive subgroups and the percentage of each who had prolonged ARS symptoms.
The results from Table 2 provide percentages of participants with prolonged ARS symptoms vs resolved ARS symptoms in the 6 subgroups. Comparing the positive and negative CRP, clinician impression/gestalt, and purulent nasal discharge subgroups, there was a higher percentage of participants with prolonged ARS in the positive subgroup compared with its negative subgroup counterpart. There was a higher percentage of participants with resolved ARS in the negative CRP, clinician impression/gestalt, and purulent nasal discharge subgroup compared with its positive subgroup counterpart. However, all 6 subgroups had a higher percentage of participants with prolonged ARS vs resolved ARS.
In Table 3, the positive observed likelihood ratios were 1.71 (CRP), 2.02 (clinician impression/gestalt), and 2.69 (purulent discharge). The negative observed likelihood ratios were 0.90 (CRP), 0.66 (clinician impression/gestalt), and 0.78 (purulent discharge).
The results from Figure 2 show the number of positive subgroups that participants were a part of and the percentage of participants with prolonged ARS. A total of 60.4% of participants with prolonged ARS were a part of at least 1 positive subgroup. However, among the possible 3, 2, 1, or 0 positive subgroups that participants could be a part of, 0 (n = 38) was the mode out of the 96 participants with PARS. As shown in Figure 2, of all 140 participants, 4 were part of all 3 positive subgroups (all had prolonged ARS); 23 were a part of 2 positive subgroups (21 of whom had prolonged ARS); 47 were a part of 1 positive subgroup (33 of whom had prolonged ARS); and 66 were a part of 0 positive subgroups (38 of whom had prolonged ARS().
Discussion
The results from Table 2 are important because they suggest that certain positive clinical findings at baseline could predict prolonged ARS. The data suggest that elevated CRP, a high/intermediate clinician gestalt, or the presence of purulent nasal discharge could be early signs of a bacterial infection. While further research and an increased sample size are needed, the findings in this substudy support claims made in previous literature.7–9 Previous literature found positive likelihood ratios greater than 1 for CRP, clinician gestalt, and purulent discharge in diagnosing acute bacterial rhinosinusitis (1.84 [CRP], 3.9 [clinician impression/gestalt], and 1.6 [purulent discharge]).7–9 The observed positive likelihood ratio was also greater than 1 in all 3 positive subgroups (1.71 [CRP], 2.02 [clinician impression/gestalt], and 2.69 [purulent discharge]). However, the confidence interval for the positive CRP group did cross 1. The data from both the literature and this study suggest that these 3 positive subgroups could be a specific measure in ruling in acute bacterial rhinosinusitis early on in disease progression. It should be noted that the likelihood ratios from previous literature were from cases of confirmed acute bacterial rhinosinusitis Additionally, the results from Figure 2 potentially suggest that the more positive subgroups that a participant was a part of, the more likely they had prolonged ARS. It should be noted that most participants were categorized as being a part of 1 or 0 positive subgroups. However, the minority that was a part of 2 or more positive subgroups represented more than 91% of participants with prolonged ARS. Furthermore, these results are important because they can act as a step toward clinicians having more clarity about when patients will have prolonged ARS symptoms. Guidelines suggest that patients with prolonged ARS symptoms greater than 10 days should be treated with antibiotics.1 Early identification of the need for antibiotics in prolonged ARS cases may have mortality and cost-efficiency benefits. Additionally, these findings can help clinicians identify who will benefit from antibiotics in the case of ARS and limit their overprescription. This is especially important because antibiotic resistance continues to be an issue plaguing society.
A major limitation of this study was the sample size. The data collected for this study were from a pilot study for a larger planned RCT. As the RCT progresses, we hope to collect similar data on a much larger scale. Having these data on a larger scale can not only confirm previous literature, but further answer the question about which subgroups/types of patients will benefit from early treatment of ARS with antibiotics. Future research should include other nonspecific clinical findings that have been associated with sinusitis.
Conclusion
Because acute bacterial sinusitis continues to be largely common, it remains important for clinicians to diagnose this infection early on and practice proper antibiotic stewardship. Previous literature and findings of this study suggest certain clinical findings and impressions early on can indicate a bacterial infection, vs viral infection, and allow for appropriate prescription of antibiotics. This study found that elevated CRP levels, the presence of purulent nasal discharge, and high or intermediate physician suspicion that the patient has acute bacterial sinusitis can potentially be a specific finding in ruling in acute bacterial sinusitis early on in disease progression. Additionally, we observed more positive findings at baseline correlated with a higher percentage of participants with prolonged sinusitis symptoms. We hope that this research acts as a step toward finding a more effective way to diagnose acute bacterial sinusitis.
Sources of Support
This study was supported by a Mitchell Summer research scholar stipend.
The randomized, placebo-controlled trial was registered with the following information
• NCT Number -NCT06076304
• PCORI 2021C3-24476
• BRANY IRB 23-02-622