Introduction
Background
Psoriasis is a chronic, immune-mediated skin condition that impacts around 125 million people globally, corresponding to about 2% to 3% of the population.1,2 In the United States alone, more than 7.5 million adults are affected.3 Epidemiological data demonstrate comparable prevalence across sexes; however, White adults exhibit 1.9 times higher odds of developing psoriasis compared with ethnic minority groups.3 Notably, socioeconomic factors, such as marital status, education level, income, and medical insurance coverage, do not appear to influence disease prevalence directly; however, these factors may contribute to barriers in accessing treatment and managing the disease.3 Common comorbidities include psoriatic arthritis, Crohn disease, metabolic syndrome, cardiovascular disease, and psychiatric disorders. Comorbidities typically emerge years after psoriasis onset and occur more frequently in patients with more severe disease.1 While knowledge of psoriasis has expanded due to ongoing research and clinical trials, significant gaps in understanding remain. Although certain genes have been identified that confer susceptibility to psoriasis, no single gene definitively determines disease presence or absence. Furthermore, while various theories exist regarding psoriasis pathogenesis, the precise mechanisms are not completely understood, with current evidence supporting the involvement of both environmental and genetic components.4
Clinical Features and Diagnostic Criteria
Psoriasis presents in several clinical forms, including plaque psoriasis, guttate psoriasis, pustular psoriasis, erythrodermic psoriasis, and psoriatic arthritis.5 Plaque psoriasis is the most common, affecting more than 80% of individuals with psoriasis worldwide.2 These lesions appear as sharply defined, symmetric, and erythematous plaques with an overlying micaceous scale and are commonly found on the scalp, and extensor surfaces of the trunk, elbows, knees, feet, and hands.6,7
Clinicians gauge psoriasis severity primarily by the percentage of body surface area (BSA) involved. Per the American Academy of Dermatology and National Psoriasis Foundation guidelines, mild disease corresponds to less than 3% BSA; moderate disease, 3% to 10% BSA; and severe disease, more than 10% BSA.8 The severity of psoriasis can also be assessed using the Psoriasis Area and Severity Index (PASI), which considers the intensity of redness, scaling, and plaque thickness, in addition to the BSA affected. The PASI score ranges from 0 (no psoriasis) up to 72 (most severe).8–10
Risk Factors
The etiology of psoriasis arises from the interaction between genetic susceptibility and environmental stimuli. Epidemiologic studies in twins have demonstrated a 65% to 72% concordance rate in monozygotic twins compared with 15% to 30% in dizygotic twins, indicating a high heritability of this condition.11 Given the autoimmune nature of this disease, it is unsurprising that human leukocyte antigen (HLA) studies have found strong associations between the HLA-Cw6, HLA-B57, and HLA-DR7 alleles and early-onset psoriasis, while HLA-Cw2 correlates with late-onset psoriasis.12 These HLA alleles play crucial roles in immune system function: HLA-Cw6 and HLA-B57 are class I HLA antigens that present foreign antigens to killer T cells, enabling immune surveillance of infected or abnormal cells, while HLA-DR7 is a class II HLA antigen that presents foreign antigens to helper T cells, facilitating adaptive immune responses. These HLA associations provide important context for psoriasis pathogenesis, as these molecules are important for antigen identification and T-cell activation processes central to the autoimmune inflammation characteristic of psoriasis.12 Additional susceptibility loci include genes affecting the skin barrier (eg, LCE3B-LCE3C), innate immunity (eg, TNFAIP3, TNIP1), and adaptive immunity (eg, IL12B, IL23R).13 However, the effect of these genes appears relatively modest when compared with variants found in the major histocompatibility complex region on chromosome 6, particularly HLA-Cw6.13
While genetic variants are the likely source of immune dysregulation in psoriasis, environmental factors play a crucial role in triggering the onset of symptoms. These risk factors can be categorized into extrinsic and intrinsic factors. Extrinsic factors include physical stressors (mechanical stress, injury to the dermis, air pollution), drugs, vaccinations, infections, and lifestyle factors (smoking and alcohol consumption). Intrinsic factors include metabolic conditions (obesity and diabetes), hypertension, and mental stressors.14
Comorbidities
Psoriasis often coexists with other diseases, notably psoriatic arthritis, cardiovascular and metabolic conditions, and psychiatric disorders. Although psoriatic arthritis is relatively rare in the general populace, as much as 41% of patients with psoriasis develop joint inflammation, pain, and stiffness.15 Regarding myocardial infarction, a large cohort study demonstrated that, after adjusting for major cardiovascular risk factors, the cardiovascular risk among patients with psoriasis varied based on the severity of the condition and the patient’s age.16 For instance, a 30-year-old patient with mild psoriasis had a relative risk of myocardial infarction of 1.29 (95% confidence interval [CI], 1.14-1.46), while a 30-year-old patient with severe psoriasis had a relative risk of 3.1 (95% CI, 1.98-4.86). Meanwhile, a 60-year-old patient with severe psoriasis had an increased relative risk of 1.36 (95% CI, 1.13-1.64) when compared with their age-matched peers without psoriasis. These findings indicate that younger patients with psoriasis had a greater relative risk of myocardial infarction compared with older patients with psoriasis.16 Similarly, both mild and severe forms of psoriasis independently increase stroke risk.17 Moreover, emerging evidence reveals the complex bidirectional relationship between psoriasis and mental health, with a large mendelian randomization study establishing significant associations with depression.18 Given the various health implications, comprehensive screening for comorbidities is crucial for effective patient management and holistic care.
Pathophysiology of Plaque Psoriasis
Immune Dysregulation and Cytokine Dysfunction
Psoriatic inflammation is typically activated by an environmental trigger that induces an immune response, which causes uncontrolled keratinocyte proliferation, acanthosis, and immune cell infiltration. While not entirely understood, one proposed mechanism centers around the activation of dendritic cells. Activated keratinocytes release antimicrobial peptides (AMPs) that activate toll-like receptors on sentinel plasmacytoid dendritic cells.19,20 These activated plasmacytoid dendritic cells begin the plaque formation through the production of type 1 interferons (IFN-α and IFN-β), which signal myeloid dendritic cells to activate the maturation and differentiation of type 1 helper T cells (Th1) and type 17 helper T cells (Th17).19 These activated T cells and antigen-presenting cells secrete tumor necrosis factor–α (TNF-α), a key cytokine in the pathogenic loop. TNF-α acts in concert with IFN-γ—secreted by Th1 cells—to upregulate endothelial chemokines and adhesion molecules, facilitating further T-cell recruitment.21 Notably, levels of IL-10, a type 2 cytokine that suppresses the immune response, are decreased in psoriatic lesions, further perpetuating the inflammatory cascade.22
The pathogenic loop is maintained primarily through the IL-23/IL-17 inflammatory pathway (target of IL-23/IL-17 inhibitors). IL-17 is produced by TH17 cells and stimulates keratinocytes to release cytokines and recruit more inflammatory cells including more cells that produce IL-17.23 Myeloid dendritic cells, which are abundant in psoriatic lesions, secrete IL-23, which promotes the survival of Th17 cells. IL-23 stimulates immune cells along with proinflammatory cytokines.23. Engagement of Janus kinase/signal transducer and activator of transcription signaling in Th17 cells leads to increased IL-17 secretion, fueling keratinocyte hyperproliferation and further AMP release, thus reinforcing the cycle.24 Given their key roles in the pathophysiology of psoriasis, TNF-α, IL-23, and IL-17 have emerged as crucial targets for precision medical therapies.
Current Therapies
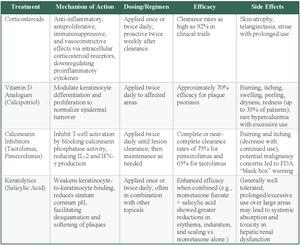
Topical Corticosteroids
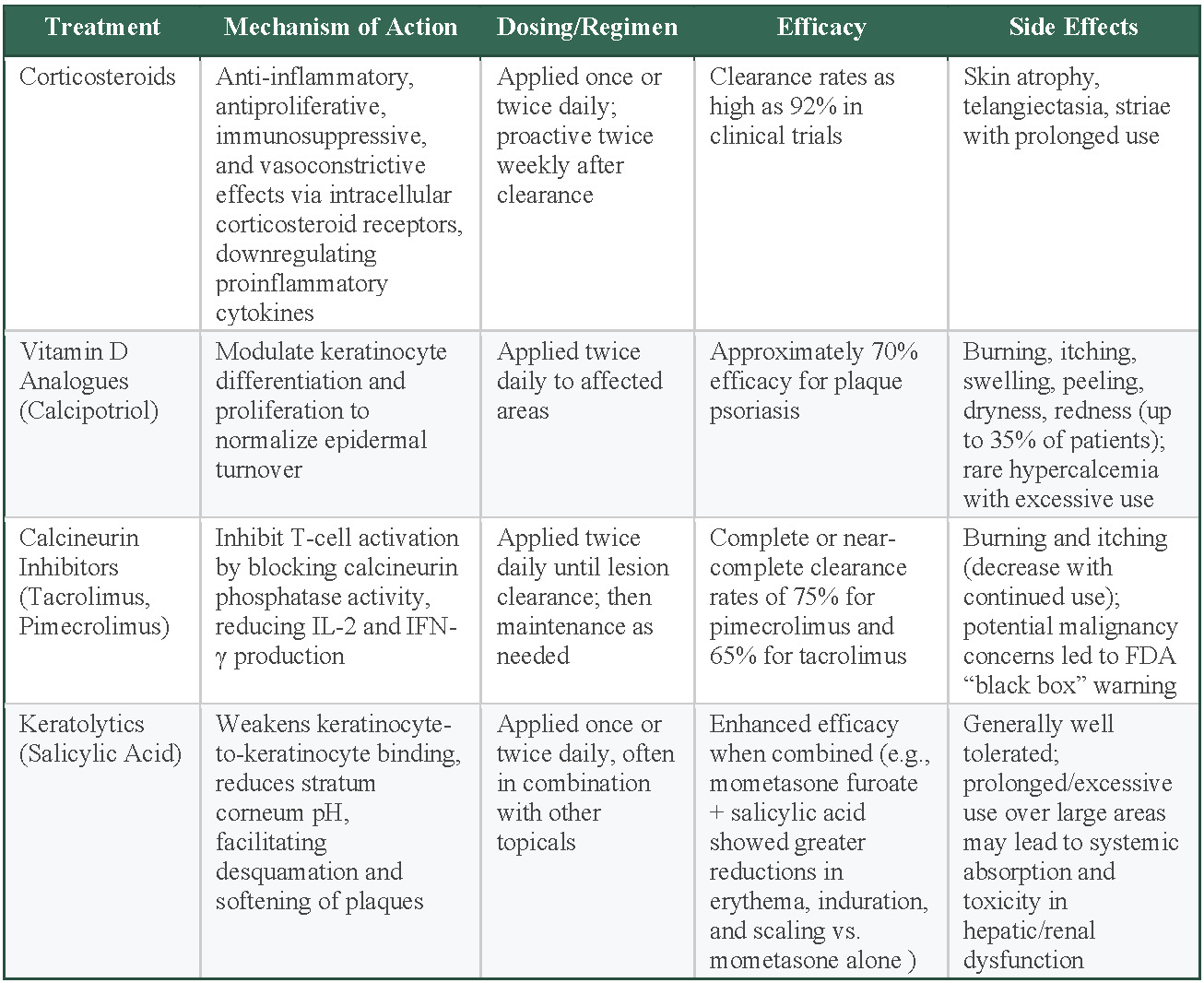
Corticosteroids are the most widely used topical treatment for psoriasis and serve as the cornerstone therapy for most patients, particularly those with limited disease. Their anti-inflammatory properties and effectiveness in reducing erythema, scaling, and pruritus make corticosteroids a primary choice for psoriasis treatment.25 They exert their action through anti-inflammatory, antiproliferative, immunosuppressive, and vasoconstrictive effects, mediated by intracellular corticosteroid receptors and gene regulation of proinflammatory cytokines.25 Corticosteroids have demonstrated strong efficacy in psoriasis treatment, with superpotent formulations achieving clearance rates as high as 92% in clinical trials.25 Typically, corticosteroids are applied once or twice daily. Some studies have also suggested proactive treatment strategies, such as twice-weekly application after lesion clearance, to sustain long-term disease control.25,26 Prolonged use, however, can result in negative effects such as skin atrophy, telangiectasia (spider veins), and striae, necessitating careful selection of potency based on lesion location and disease progression.25 Lower-potency corticosteroids are recommended for use on the face and skin folds (intertriginous areas), while higher-potency agents are reserved for thicker plaques on the trunk and extremities.25 To minimize these adverse effects, topical steroids are often prescribed in cycles that provide patients with 2 steroid-free weeks each month.27 If a patient does not achieve clearance with this regimen, escalation to systemic treatments or biologics is considered.27
Topical Vitamin D Analogs
Vitamin D analogs, such as calcipotriol (also known as calcipotriene in the United States), function by modulating keratinocyte differentiation and proliferation, thereby normalizing epidermal turnover.26 Clinical evidence suggests that calcipotriene is highly effective, with an observed efficacy rate of 70% in treating plaque psoriasis.25 The standard dosing regimen for calcipotriene is twice daily to the affected areas. Approximately one-third of individuals who use calcipotriene may experience adverse effects at the application site, including irritation, itchiness, inflammation, flaking, dryness, and redness. To enhance efficacy and minimize adverse effects, calcipotriene is often used in combination with topical corticosteroids.28 In short-term trials of 4 weeks’ duration, which included 1603 patients with mild to severe psoriasis, 48% of patients treated with the combination of calcipotriene 0.005% ointment and betamethasone dipropionate (a topical corticosteroid) 0.064% ointment once or twice daily achieved an end point of absent to mild disease, which compared favorably vs patients treated with either calcipotriene alone (16.5%) or betamethasone alone (26.3%).28 Hypercalcemia, though rare, has been observed in cases of excessive use of topical vitamin D analogs (> 100 g/wk), particularly when applied to large body surface areas or patients with underlying kidney pathology or disrupted calcium processing.25 Vitamin D analogs are often chosen as a steroid-sparing option or as adjuncts for maintenance therapy, especially when patients cannot tolerate prolonged corticosteroid use.29
Topical Calcineurin Inhibitors
Calcineurin inhibitors, such as tacrolimus and pimecrolimus, are immunomodulators that inhibit T-cell activation by blocking calcineurin’s phosphatase activity. This leads to decreased IL-2 and IFN-γ production, thereby reducing inflammation.26 These agents show particular efficacy for psoriasis in areas, such as the face and intertriginous regions (skin folds, such as the groin or underarms), where corticosteroids are not ideal due to the risk of skin atrophy.25 Dosing typically involves twice-daily application until lesion clearance.30 After clearance, a maintenance regimen of application 2 to 3 times weekly to previously affected sites may be considered to reduce the risk of relapse. The duration of both initial and maintenance therapy is at the physician’s discretion, based on patient response and clinical judgment.30 After 8 weeks of twice-daily application of pimecrolimus to intertriginous psoriasis, one double-blind, randomized clinical trial (RCT) reported that 75% of patients attained total or near-total lesion resolution.31 Another double-blind RCT reported a 65% complete or near-complete clearance rate of facial and intertriginous psoriasis following 8-week tacrolimus therapy.32 The most common adverse effects were burning and itching sensations, which lessened with continued usage.25 These adverse effects can be mitigated by prior corticosteroid use or not applying immediately after bathing. The long-term safety of calcineurin inhibitors remains uncertain. Due to concerns regarding a potential association with malignancy and the systemic formulations of calcineurin inhibitors, the US Food and Drug Administration (FDA) implemented a black box warning in 2005 for tacrolimus and pimecrolimus.33 The warning was based on animal data with systemic formulations, and the risk with topical therapy appears to be extremely low in clinical practice.33
Topical Keratolytics
Salicylic acid is a widely used keratolytic agent in the topical treatment of psoriasis. The complete and precise mechanism remains unclear, though it is believed to function through the disruption of intercellular keratinocyte adhesion and making the stratum corneum more acidic, consequently promoting desquamation and plaque softening in psoriatic lesions.25 Salicylic acid has demonstrated significant efficacy in the treatment of psoriasis, particularly when used in combination therapies. A study comparing 408 patients with moderate to severe psoriasis concluded that a combination of mometasone furoate (a topical corticosteroid) and salicylic acid resulted in greater reductions in erythema, induration, and scaling compared with mometasone furoate alone, supporting the enhanced therapeutic benefit of incorporating salicylic acid with topical steroid therapy.34 The standard regimen involves once- or twice-daily application, particularly in hyperkeratotic plaques.26 Though generally well-tolerated, prolonged or excessive use—especially over large body areas—with these topical agents can result in systemic absorption and toxic accumulation, which may pose risks in individuals with hepatic or kidney dysfunction.25
Selecting the most appropriate topical treatment requires diligent consideration of factors including severity, location, and duration of usage. Corticosteroids are the most accessible first-line therapy, with up to 92% clearance rates but risk adverse side effects with prolonged use, especially on sensitive areas.25 Vitamin D analogs achieve 70% efficacy for body lesions and may be better for milder cases of plaque psoriasis.25 Calcineurin inhibitors are preferred for facial and intertriginous areas despite higher cost and FDA warnings.25 Salicylic acid serves as a treatment to primarily target cases of psoriasis with thick plaque.26
Systemic Biologic Therapies
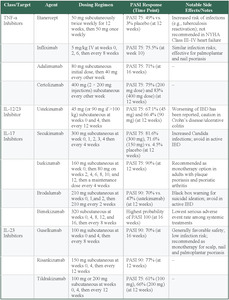
TNF-α Inhibitors
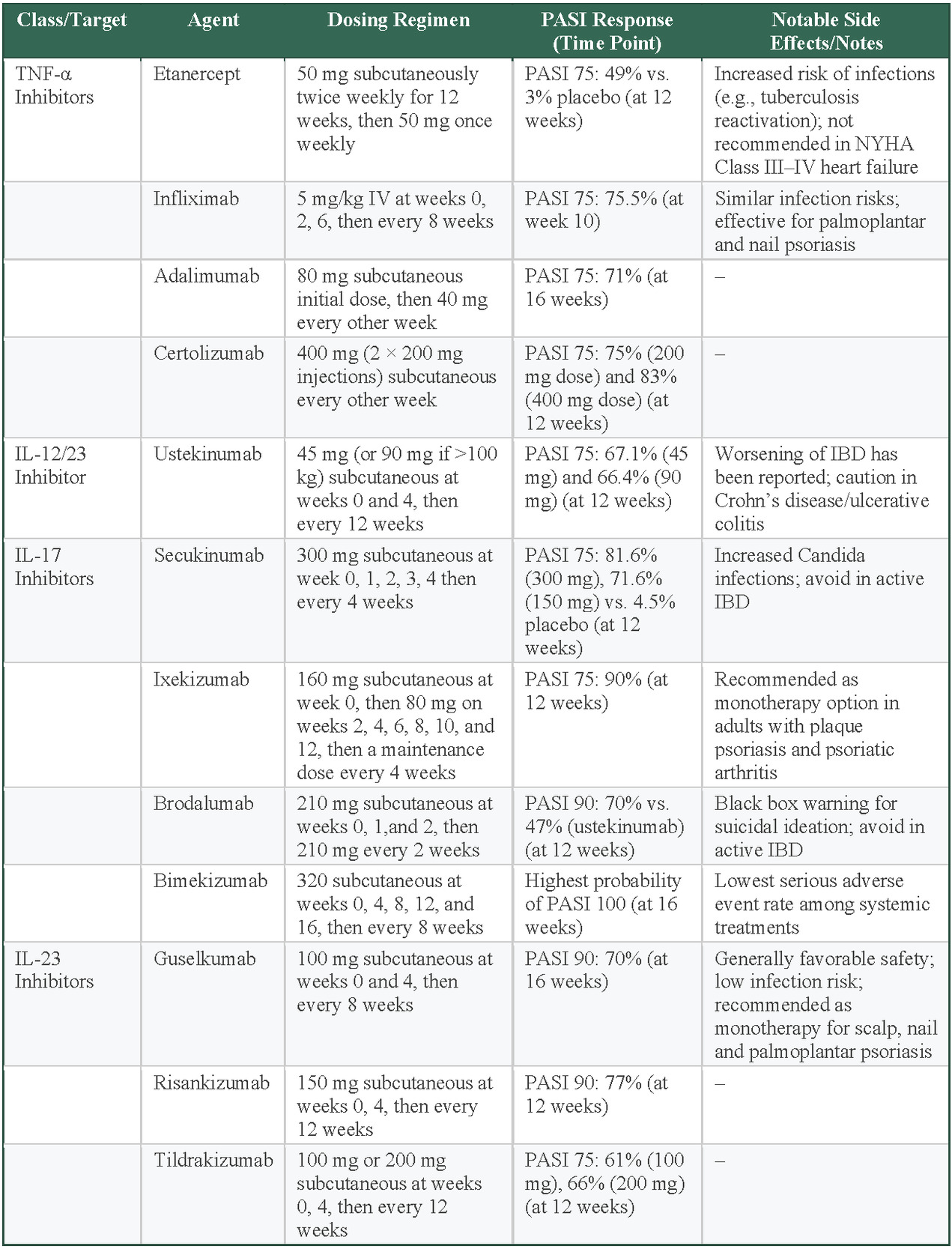
TNF-α inhibitors were the first class of biologics approved for psoriasis, providing a major leap in treating moderate to severe psoriasis. Etanercept was the first TNF-α inhibitor approved by the FDA in 2004, followed by infliximab, adalimumab, and, more recently, certolizumab.35 Etanercept is administered subcutaneously at a dose of 50 mg twice weekly for the first 12 weeks, followed by 50 mg weekly thereafter. Infliximab is administered intravenously at a dose of 5 mg/kg at weeks 0, 2, and 6 and every 8 weeks thereafter. Adalimumab is administered subcutaneously with an initial dose of 80 mg, followed by 40 mg every other week. Certolizumab is administered subcutaneously at 400 mg every other week. These drugs inhibit TNF-α, effectively reducing immune overactivation and normalizes the rate of skin cell turnover.35 Several clinical trials have concluded that TNF-α inhibitors are effective at reducing psoriasis symptoms.
An analysis of placebo-controlled RCTs of etanercept found significant reductions in psoriasis severity at 12 weeks. A 75% reduction in PASI scores (PASI 75) was achieved in 49% of patients receiving etanercept compared with only 3% in those receiving a placebo.36 Infliximab is also a well-established treatment for patients with moderate to severe psoriasis. In a pivotal phase 3 study, infliximab achieved PASI 75 in 75.5% of patients at week 10 of treatment.37 Furthermore, infliximab is effective in treating plaque-type palmoplantar and nail psoriasis.35 Adalimumab achieved PASI 75 in 71% of patients at 16 weeks.35 Certolizumab also achieved PASI 75 in 75% (200-mg dose) and 83% (400-mg dose) of patients at 12 weeks.35
While TNF-α inhibitors are effective, they can carry some risks. Patients taking these drugs have a higher risk of infections, including reactivation of tuberculosis, so regular screening for latent tuberculosis (using a purified protein derivative test or IFN-γ release assay), hepatitis B (with assessment and immunization if needed), and other infections is recommended before and during treatment.38 They are also costly, with prices reaching up to $30 000 per year, not including the cost of administration, and access may be limited by insurance coverage.38 Additionally, these medications are not recommended for patients with advanced heart failure.39 Clinical trials in advanced heart failure have found that broad TNF-α blockade can impair cardiac function.39 This is thought to arise because TNF-α has both protective (via TNFR2/TRAF2 signaling and membrane-bound forms) and harmful (via TNFR1 and secreted TNF-α) effects on the myocardium, and indiscriminate inhibition disrupts compensatory remodeling pathways.39 As a result, TNF-α inhibitors are contraindicated in patients with moderate to severe heart failure.39
Interleukin-12/23 Inhibitors
Interleukin-12/23 (IL-12/23) inhibitors target the shared p40 subunit of IL-12 and IL-23, blocking key pathways that contribute to psoriasis.35 This action reduces inflammation and slows keratinocyte proliferation. Ustekinumab (an IL-12/23 inhibitor biologic) produced a mean 81.9% improvement in PASI score at 12 weeks. By week 52, 92.2% of patients taking ustekinumab achieved PASI 75 and 44.8% achieved PASI 100.40 The recommended dosing starts with 45 mg (or 90 mg for patients weighing >100 kg) at weeks 0 and 4. Maintenance doses are then administered every 12 weeks. While the safety profile of IL-12/23 inhibitors is generally positive, with a lower rate of immune-related adverse effects compared with TNF-α inhibitors, they are associated with a risk of mild to moderate adverse events—most commonly infections—along with occasional serious events such as malignancies, serious infections, or cardiovascular complications.35,40
IL-17 Inhibitors
IL-17 inhibitors are a highly effective class of biologics for psoriasis. Given that IL-17A is a crucial cytokine in the pathophysiology, its blockade greatly reduces keratinocyte proliferation and inflammation, thereby reducing disease severity.35 Among IL-17 inhibitors, secukinumab has demonstrated PASI 75 response rates of 81.6% in patients receiving 300 mg, 71.6% in those receiving 150 mg, and 4.5% in the placebo group at 12 weeks.35 Ixekizumab has shown greater efficacy, with 90% of patients achieving PASI 75 in the same period.35 Brodalumab, an IL-17 receptor A blocker, reached a 90% reduction in PASI (PASI 90) in 70% of patients compared with 47% for ustekinumab at week 12.35 IL-17 inhibitors have been associated with some of the highest PASI 90 and PASI 100 response rates among all biologics, making them a leading option for achieving near-complete skin clearance.41 A systematic review of 77 trials concluded that IL-17 inhibitors, particularly brodalumab and ixekizumab, perform comparably vs IL-23 inhibitors and surpass TNF-α inhibitors in achieving PASI 90 and PASI 100.42 Bimekizumab, which targets both the IL-17A and IL-17F isoforms, has been shown to have the highest probability of patients achieving PASI 100 after 16 weeks of treatment for plaque psoriasis, while also having the lowest serious adverse effect rate of all systemic treatments.43,44
Despite their strong efficacy, IL-17 inhibitors carry certain risks. They have been linked to increased rates of Candida infections, as IL-17 plays a role in antifungal immunity.45 Additionally, these agents may exacerbate Crohn disease and should be avoided in patients with active inflammatory bowel disease.35 Brodalumab carries a black box warning due to rare reports of suicidal thoughts, necessitating careful patient monitoring.35
IL-23 Inhibitors
IL-23 promotes the survival of Th17 cells, which drives keratinocyte hyperproliferation through IL-17 production and the release of AMPs.24 IL-23 inhibitors block the p19 subunit of IL-23, making them more selective than IL-12/23 inhibitors.35 Guselkumab (an IL-23 inhibitor biologic) has demonstrated PASI 90 in 70% of patients at 16 weeks, with a dosing regimen of 100 mg subcutaneously at weeks 0 and 4, followed by every 8 weeks.35 Risankizumab (also an IL-23 inhibitor biologic) achieved PASI 90 in 77% of patients at 12 weeks with 150-mg doses at weeks 0 and 4 and every 12 weeks thereafter.35 Tildrakizumab (another IL-23 inhibitor) reached PASI 75 in 66% (200 mg) and 61% (100 mg) of patients at 12 weeks, with dosing at 0 and 4 weeks and every 12 weeks.35 IL-23 inhibitors offer a reduced risk of infections and have not shown unique adverse events of interest.35 They also provide strong long-term disease control, making them an attractive option for patients requiring extended psoriasis management.35
Selecting the most appropriate biologic therapy for patients with moderate to severe psoriasis requires careful consideration of several patient-specific factors. Insurance coverage and cost play significant roles, as biologic treatments can be costly. TNF-α inhibitors can cost more than $30 000 annually, potentially limiting accessibility based on insurance plans.46 Concurrent medical conditions should also guide the selection; IL-17 inhibitors, for example, should be avoided in patients with active inflammatory bowel disease due to potential exacerbation.35 Conversely, infliximab (a TNF-α inhibitor) may benefit patients presenting with plaque-type palmoplantar or nail psoriasis.35 Contraindications must also be evaluated carefully; TNF-α inhibitors are contraindicated in patients with moderate to severe heart failure due to adverse cardiac remodeling effects.39 Additionally, patient adherence and preferences regarding injection frequency must be assessed. Agents such as ustekinumab offer a convenient dosing schedule every 12 weeks after initial loading doses, whereas others such as etanercept or certolizumab require more frequent administration.35,43 Last, potential adverse events—including increased infection risk with TNF-α and IL-17 inhibitors, Candida infections specifically with IL-17 inhibitors, and rare psychiatric events with brodalumab—should be considered and monitored appropriately.35,45
Systemic Nonbiologic Therapies
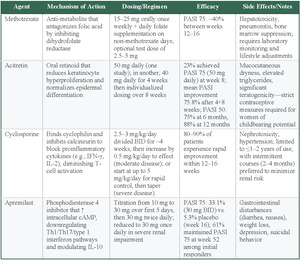
Methotrexate
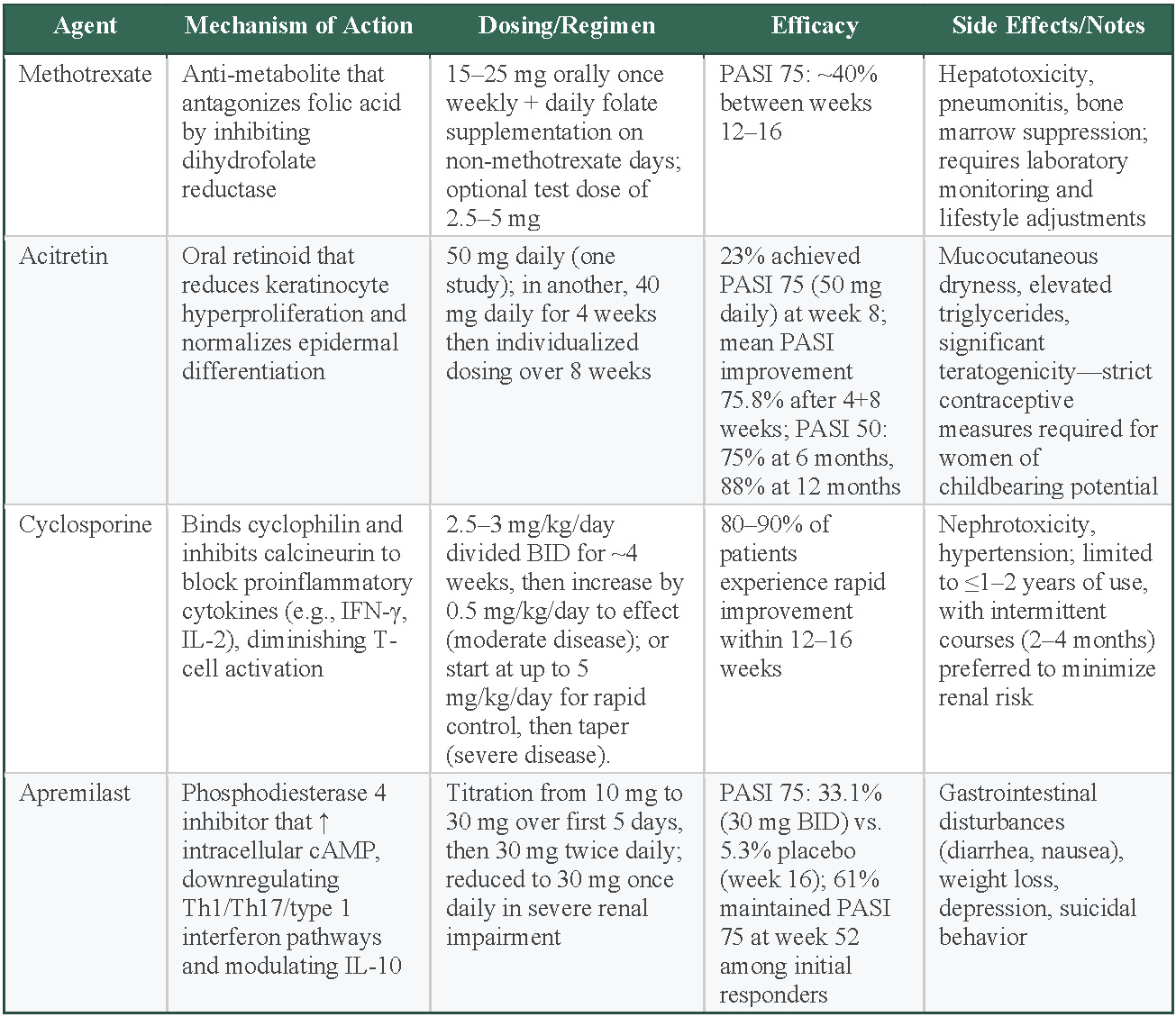
Methotrexate is an antimetabolite that antagonizes folic acid by inhibiting dihydrofolate reductase, which inhibits DNA synthesis and suppresses the proliferation of rapidly dividing cells, including activated immune cells and keratinocytes involved in psoriasis pathogenesis.47 This agent is typically administered orally between 15 and 25 mg weekly along with daily folate supplementation on days when methotrexate is not given. Patients can also be started with an initial dose of either 2.5 or 5 mg if there is concern for bone-marrow suppression.48 Meta-analyses have reported PASI 75 rates of approximately 40% between 12 and 16 weeks, while maintaining one of the lowest serious adverse event rates of all systemic treatments.44,49 However, methotrexate does carry some significant safety concerns, including hepatotoxicity, pneumonitis, and bone marrow suppression, which require laboratory monitoring and lifestyle changes.50 Methotrexate is often chosen when biologics are contraindicated, unaffordable, or unavailable or when a patient does not respond adequately to other therapies. It has also been studied as an adjunct to biologics, particularly TNF-α inhibitors adalimumab and infliximab, to suppress the production of antidrug antibodies.37–50
Acitretin
Acitretin, an oral retinoid, primarily exerts its effect by reducing keratinocyte hyperproliferation and promoting normalization of epidermal differentiation; its precise mechanism, however, is not completely understood.51 This agent appears to be especially beneficial in pustular and erythrodermic forms of psoriasis, though it may not show as much efficacy compared with other systemic medications.48 During clinical trials, various dosing regimens of acitretin have demonstrated considerable improvements: one study reported that 23% of patients receiving 50 mg daily reached a 75% improvement in PASI scores by week 8, while another trial showed a mean PASI improvement of 75.8% following an initial 4-week course of 40 mg daily followed by individualized dosing over an additional 8 weeks.52,53 Moreover, maintenance therapy yielded a 50 % reduction in PASI score in 75% of patients at 6 months and 88% at 12 months48 Acitretin utilization is restricted by an adverse effect profile, which includes mucocutaneous dryness, elevated triglyceride levels, and significant teratogenic risks that necessitate strict contraceptive measures for women of childbearing potential.51
Cyclosporine
Cyclosporine, a potent immunosuppressant approved by the FDA in 1997, acts by binding to cyclophilin and subsequently inhibiting calcineurin, thereby blocking key proinflammatory signals and reducing cytokines, much like IFN-γ and IL-2, which diminishes T-cell activation.48 Clinical data indicate that 80% to 90% of patients with plaque psoriasis experience a marked enhancement within 12 to 16 weeks of treatment.51 The utility of cyclosporine is weakened by its safety concerns; prolonged use is restricted due to risks of nephrotoxicity and hypertension. As a result, treatment durations are generally limited to 1 to 2 years with a preference for intermittent regimens, which are typically 2 to 4 months, to minimize kidney complications.51
Apremilast
Apremilast exerts its therapeutic effects by inhibiting phosphodiesterase 4, leading to an increase in cyclic adenosine monophosphate within the cell. This rise in cyclic adenosine monophosphate creates inflammatory activity reduction mediated by T helper 1 (Th1), type 1 IFN pathways, and Th17, while also modulating anti-inflammatory cytokines, amongst them, IL-10, thereby improving the overall inflammatory profile in psoriasis.48 An RCT containing 844 patients found that participants treated with 30 mg of apremilast twice daily achieved a PASI 75 response rate of 33.1% at week 16 compared with 5.3% in the placebo group.48 After week 16, a portion of those in the placebo and treatment groups were re-randomized and followed up through week 52.48 Of those who continued the therapy, 61.0% of those who initially responded maintained the PASI 75 response at week 52, whereas a significant proportion of participants who were re-randomized to placebo lost their response and required reinitiation of therapy.48 The dosing regimen involves a gradual titration starting at 10 mg daily with increments of 10 mg per day over the first 5 days, reaching a maintenance dose of 30 mg twice daily; a dose of 30 mg once daily is given to patients with reported severe kidney impairment.48 Reported adverse effects include gastrointestinal disturbances such as diarrhea and nausea, as well as weight loss, depression, and suicidal behavior.54
Methotrexate offers moderate efficacy (40% PASI 75), with the lowest serious adverse event rates among systemic treatments, and is typically the first-line choice for systemic nonbiologic therapies.44,49 However, this requires lifestyle changes and monitoring, which may serve as a barrier to some. Acitretin shows variable efficacy (23% of patients achieving PASI 75) with particular benefit for pustular and erythrodermic psoriasis, but is limited by mucocutaneous adverse effects, elevated triglycerides, and strict teratogenic precautions for women.52,53 Cyclosporine provides the highest efficacy (80%-90% of patients having a marked improvement) with rapid onset within 12 to 16 weeks, but nephrotoxicity and hypertension risks limit use to intermittent 2- to 4-month courses over 1 to 2 years maximum.51 Apremilast offers modest efficacy (33% PASI 75) with oral convenience and gradual titration dosing, but gastrointestinal adverse effects, weight loss, and depression concerns may limit tolerability.48
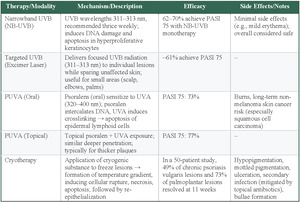
Phototherapy
Phototherapy is a common nonpharmacologic treatment for moderate to severe plaque psoriasis when topicals alone are insufficient but systemic therapy is undesired. It may be used independently or as an adjunct to topical treatments.55 Several modalities and regimens can be used, each with distinct advantages and limitations, including narrowband ultraviolet B (NB-UVB), psoralen ultraviolet A (PUVA), targeted UVB treatments such as excimer laser, and Grenz ray therapy.55
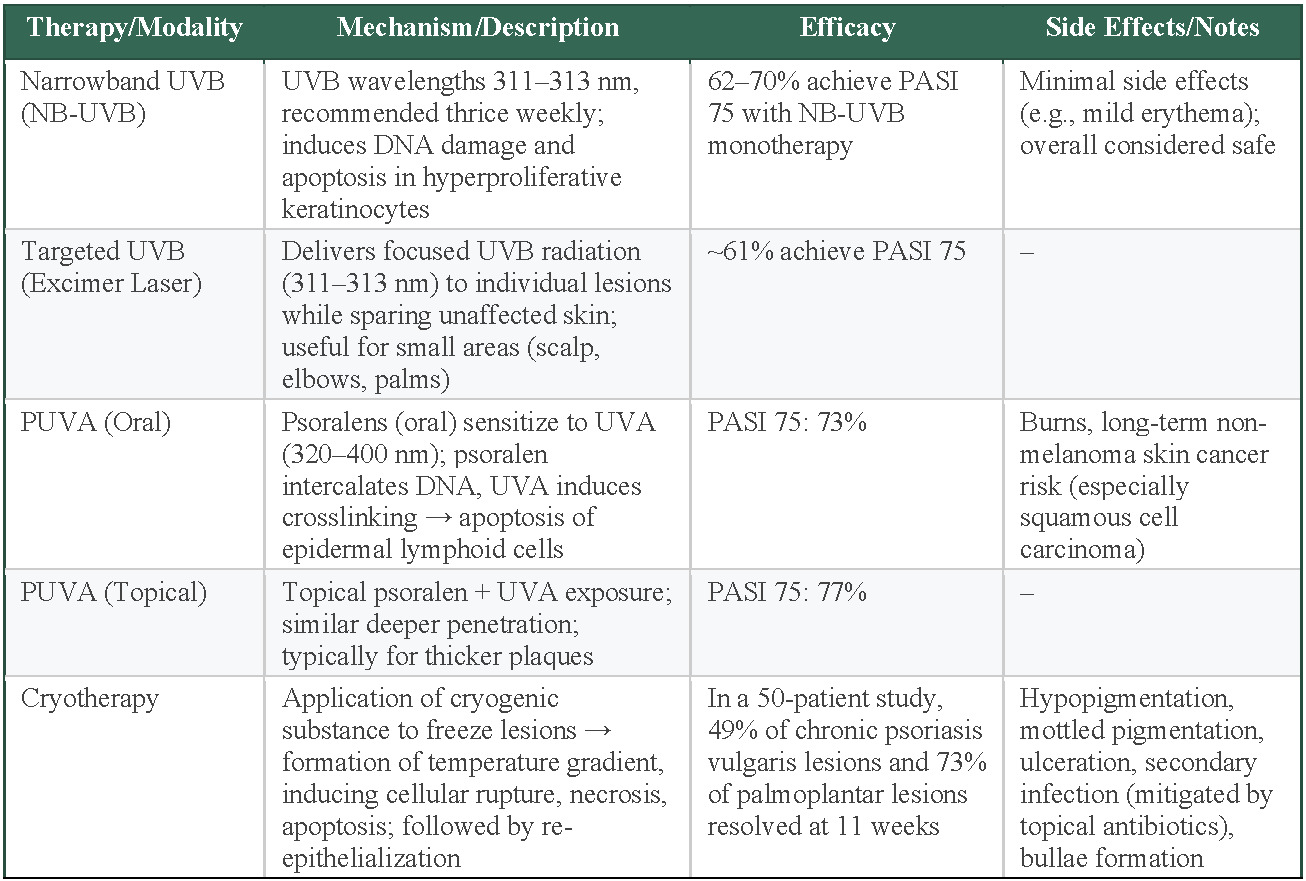
NB-UVB is the most commonly used form of phototherapy, especially for generalized plaque psoriasis. It uses 311- to 313-nm TL-01 lamps and is recommended 3 times per week; however, dosing regimens in the literature range from twice weekly to 5 times weekly, with total sessions (averaging around 25) adjusted based on factors such as minimal erythemal dose testing, Fitzpatrick skin type, and protocol-specific dose increments.56,57 NB-UVB is considered to be a safe and effective option, with meta-analyses reporting that 62% to 70% of patients achieve PASI 75 following NB-UVB monotherapy and have minimal adverse effects.56,57 Targeted UVB, or excimer laser, delivers a dose of UVB radiation (wavelength, 311-313 nm) to an individual lesion, while excluding unaffected skin. This treatment is especially useful for small areas, such as the scalp, elbows, or palms, and can result in rapid improvement.55 An estimated 61% of patients receiving targeted UVB achieved PASI 75. While this result may not seem as impressive as other phototherapies, the benefits of precision treatment, faster clearing, and lower overall UV exposure must be considered.
PUVA is often used in patients with thicker and more localized plaques. Psoralens are topical or oral agents that sensitize target cells to the effects of UVA wavelengths between 320 and 400 nm. Following UVA exposure, psoralens prevent DNA replication in hyperproliferative keratinocytes, induce apoptosis, and promote depletion of pathogenic lymphoid cells.55 This approach penetrates deeper into the skin and is typically reserved for resistant or more severe plaques. In terms of efficacy, PASI 75 was achieved in 73% of patients receiving oral PUVA in one meta-analysis and 77% of patients receiving topical PUVA in another.56,58 Prolonged use can cause harmful burns and has been linked to an elevated risk of nonmelanoma skin cancers, in particular squamous cell carcinoma, limiting its use as a long-term maintenance option.55
Based on these phototherapy modalities, selection depends on disease extent, lesion characteristics, and safety considerations. NB-UVB is the most commonly used option for generalized plaque psoriasis, achieving 62% to 70% PASI 75 response with minimal adverse effects, making it the safest long-term phototherapy choice.56,57 Targeted UVB provides precision treatment for localized areas such as the scalp, elbows, or palms, achieving 61% PASI 75 response with benefits of faster clearing and reduced overall UV exposure to unaffected skin.55 PUVA therapy offers the highest efficacy (73%-77% PASI 75) for thicker, resistant plaques, but prolonged use risks burns and increased nonmelanoma skin cancer risk, particularly squamous cell carcinoma, limiting long-term use.56,58 Treatment selection prioritizes NB-UVB for generalized disease and long-term safety, targeted UVB for localized lesions requiring precision, and PUVA for resistant plaques when higher efficacy outweighs cancer risks.
Interventional and Emerging Therapies
Noninvasive Therapies—Cryotherapy
Cryotherapy, or cryosurgery, has a long history of use in treating benign, premalignant, and malignant dermatologic conditions. Modern techniques involve applying a cryogenic substance either directly or indirectly to freeze the skin lesions. Between the affected surface layer and deeper layers, a temperature gradient is formed that correlates with zones of cellular rupture, necrosis, and apoptosis.59 Proposed mechanisms for the efficacy of cryotherapy include normal re-epithelialization after the destruction of the psoriatic lesion, shortening of the elongated dermal papillae, and the creation of an altered dermis that is less hospitable to immune cells.60 One early self-controlled prospective cryotherapy study reported a complete lesion resolution rate 56% in 9 patients at 11 weeks after receiving one treatment.60 This study used a split body design where lesions of approximate size and severity were matched on both sides of the participants body and randomly assigned as treatment or control. Despite the small sample size, the design of this study was an improvement over and an earlier uncontrolled study by Robert Scoggins.61 This landmark study by Scoggins established that bullae formation is crucial for cryotherapy to be effective and he found that 67% (31 plaques treated in 12 patients in the preliminary 1985 trial) and 80% (191 lesions treated in 23 patients in the subsequent 12-month study) achieved complete resolution after a single treatment.61 More recently, a 2024 study of 50 patients with chronic psoriasis vulgaris and palmoplantar psoriasis used the split-body randomized self-controlled design treating 183 lesions with cryotherapy and 160 lesions with a bland emollient. These researchers found that at 11 weeks, 49% of the psoriasis vulgaris lesions and 73% of palmoplantar lesions completely resolved.62
While this 49% is lower than prior studies, it is likely more accurate due to the larger sample size and improved study design. However, the 11 week follow up limits our understanding of this durability of this result. The superior response of the palmoplantar lesions likely reflects anatomical and pathophysiological differences: the thicker stratum corneum may allow for deeper penetration without damaging underlying structures, while the absence of hair follicles in these areas could reduce inflammatory mediators that might otherwise trigger recurrence.
Common adverse effects of cryotherapy include, but are not limited to, hypopigmentation, mottled pigmentation, ulceration, and secondary infection, which can be avoided with the use of topical antibiotics.61–63 Bullae can also form, but early studies suggest that this is necessary for successful resolution.61 A significant limitation of cryotherapy is that the treatment itself can be acutely painful, making it most useful for small, stubborn plaques that do not resolve with topical therapies.62
Minimally Invasive Therapies—Microneedles
Microneedles are micron-sized needles that are typically arranged in a grid that projects perpendicularly into the skin. This technique aims to improve the efficacy and durability of local treatments, while minimizing the risks associated with systemic treatments. Microneedle diameters between 5 to 37 µm with lengths greater than 600 µm are ideal for penetrating the tough stratum corneum and providing access to deeper layers of the epidermis and dermis while reducing traumatic disruption of the skin barrier.64,65 Microneedles can be categorized into the following types: solid, hollow, dissolving, coated, and hydrogel forming.66 Solid microneedles create micropores into which topical therapies are applied while hollow microneedles allow for drugs to be applied directly through a channel in the needle.67 Coated microneedles are layered with a therapeutic agent that diffuses into the surrounding interstitium, while dissolving microneedles are fully absorbed deep into the skin.67 Hydrogel-forming microneedles are unique in that they swell when in contact with interstitial fluid, effectively anchoring the therapeutic agent and allowing precise release of the drug.68 There is limited evidence in humans, but several preclinical trials have suggested a potential for microneedles in psoriasis.
Many of these trials used an imiquimod-induced model of psoriasis in mice that activate the IL-17/IL-23 axis. The preclinical evidence has shown promising results across various microneedle formulations. Dissolvable clobetasol (a corticosteroid) microneedle patches demonstrated rapid effectiveness, though without significant PASI score advantages over traditional topical applications.69 Similarly, dissolvable methotrexate microneedle patches achieved equivalent efficacy at lower doses (13.8 μg per patch) compared with oral administration (27.6 μg per animal), with notable downregulation of inflammatory cytokines IL-17 and IL-23 without hepatic or kidney complications.70 However, it is important to mention that dosing protocols in mice studies are not standardized and may not directly correlate to dosing in humans. Photothermal responsive hydrogel anti–IL-17 microneedle patches significantly reduced multiple inflammatory markers including IL-23a, IL-17a, IL-22, INF-γ, and IL-6.71 Coated cyclosporine (an immunosuppressant drug) microneedle arrays showed superior PASI improvement compared with oral solutions while reducing inflammatory cytokines and improving systemic bioavailability.72 Dissolving tacrolimus (a calcineurin inhibitor immunosuppressant) nanocrystals separated within minutes, dissolved within hours, and resolved symptoms faster than ointment formulations, with significant improvements in PASI scores and decreased levels of TNF-α, IL-17A, and IL-23.73 In human studies, a self-controlled 25-patient randomized clinical trial for nail psoriasis using dissolvable triamcinolone (a corticosteroid and standard treatment for nail psoriasis) demonstrated more sustained and greater improvement in Nail Psoriasis Severity Index scores at 4 months compared with control treatments, proving equally effective as topical calcipotriol/betamethasone dipropionate combinations.74 It is likely that these treatments will be limited to small and milder lesions until more human trials validate their efficacy, safety, and practicality for widespread clinical application.
Emerging Noninvasive Therapies—Electrotherapy
The rationale for the use of electric currents in psoriasis stems from the effect of electrostimulation on inflammatory microenvironments, the potential for improved transdermal drug delivery, the effect of electric stimulation on wound healing, and the potential for at-home wearable medical devices.
In the context of drug delivery, iontophoresis has gained attention in the treatment of psoriasis. Iontophoresis uses a low voltage direct current to enhance the movement of charged molecules through the skin.75 This technique has been explored as a method to deliver therapeutic agents in a few preclinical models. Iontophoretic administration of etanercept was found to inhibit IL-6 and significantly reduce epidermal hyperplasia.76 Similarly, iontophoretic delivery of tacrolimus-loaded anionic liposomes reduced TNF-α and IL-6 to 70% and 26% of their baseline values, respectively, within 1 hour of treatment.77 When compared with tacrolimus ointment applied for 1 hour, the tacrolimus liposome iontophoresis showed significant reductions in these inflammatory cytokines, leading the authors to conclude that this technique is twice as efficacious as a topical application.77
Since the discovery that an electrical potential exists across a skin wound, researchers have sought ways to enhance wound healing using direct current electric fields.78,79 An early pilot study examined the effects of low-voltage electric fields on 6 patients with chronic treatment-resistant plaque psoriasis.80 This study used a device that generated currents between 25 and 30 mA at 25 to 30 V that travelled across the patient’s skin from their feet to their hands. Each patient underwent several treatment sessions over 3 months. The investigator found that 5 of the 6 patients achieved complete remission at 3 months, a 95% decrease in PASI scores, and no signs of recurrence at 9 months.80 Despite this promising result, this study was never replicated due to the lack of a clear mechanism and a shift in focus towards targeted therapies (eg, TNF-α inhibitors).
More recently, a preclinical study found that electrostimulation reduced psoriasis recurrence by downregulating potassium channel Kv1.3 on T cells and reducing concentrations of circulation CD4+/CD8+ effector memory and CD8+ skin-resident memory T cells.81 One in vitro study reported a suppression of Th1, Th17, and Treg-associated cytokines, with Th17 being the most sensitive to electric fields.82 Another study demonstrated that pulsed electric stimulation suppressed keratinocyte proliferation while inducing keratinocyte differentiation.83 These findings suggest potential for therapeutic effect, but without a unified framework or robust human data, it is premature to draw clinical conclusions. Additionally, questions remain about optimal electrical parameters, treatment protocols, long-term safety, and how these approaches might compare with or complement established psoriasis therapies.
Repurposed Pharmacologic Agents
Primidone
Primidone, an anticonvulsant medication approved by the FDA for epilepsy, has emerged as a potential innovative treatment for psoriasis through drug repurposing. Primidone selectively inhibits the enzymatic activity and phosphorylation of receptor-interacting serine/threonine-protein kinase 1 (RIPK1), a critical mediator of necroptosis (a highly proinflammatory form of regulated cell death).84 By blocking RIPK1 activation, primidone effectively reduces cytokine release and dampens macrophage and dendritic cell-driven inflammatory cascades without causing profound immunosuppression, a common limitation of existing biologics and immunosuppressants.85
In preclinical imiquimod-induced mice models, oral primidone administered via drinking water at a concentration of 2.875 mM significantly reduced skin inflammation symptoms and histological markers of disease severity.86 Primidone markedly blunted the daily rise in PASI scores by approximately 35% to 40% compared with controls in both preventive and therapeutic trials.86 This reduction was statistically significant from day 2 onward (P < .05) in the preventive trial and from day 3 onward in the therapeutic trial out of 5 days total.86 Furthermore, primidone effectively decreased systemic inflammation, as evidenced by notable reductions in spleen size and proinflammatory cytokine plasma levels.86 Specifically, primidone administration reduced imiquimod-induced elevations in IL-6 (~150 pg/mL to ~50 pg/mL, P < .01), IL-17A (~130 pg/mL to ~20 pg/mL, P < .05), and IL-22 (~60 pg/mL to ~10 pg/mL, P < .01).86 While primidone’s safety profile is well-established for epilepsy, its repurposing for psoriasis necessitates validation through dedicated clinical trials. Nevertheless, primidone represents a promising corticosteroid-sparing strategy, especially for patients with severe inflammation, inadequate response, or resistance to current therapies, as well as potentially synergizing with existing biologic medications to minimize dosages and adverse effects.
Semaglutide
Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist approved for type 2 diabetes and obesity, is being explored for psoriasis treatment due to its metabolic and anti-inflammatory properties.87 While GLP-1 analogs are primarily recognized for their insulinotropic effects, they also exhibit a range of extrapancreatic actions that may be therapeutically relevant in chronic inflammatory conditions such as psoriasis.87 These include suppression of cell proliferation, inhibition of macrophage migration, and impairment of inflammation through activation of adenosine 5’-monophosphate-activated protein kinase.88,89
GLP-1 analogs further increase circulating invariant natural killer T cells (a subset of innate T cells with potent immunoregulatory functions) potentially contributing to the downregulation of inflammatory responses.88,89 Importantly, GLP-1 receptor agonists may decrease dermal gamma delta (γδ) T-cell numbers and reduce IL-17 expression in psoriatic plaques, directly targeting cytokine pathways central to psoriasis pathogenesis.89 These immunomodulatory activities appear to be relevant not only for glycemic control, but also for the management of diabetes, obesity, and psoriasis, disorders that share a chronic inflammatory basis.87 Notably, clinical evidence with liraglutide, another GLP-1 analog, has shown that once-daily injection can reduce psoriasis severity in patients with type 2 diabetes, with an effect that is independent of both weight loss and glycemic control.90 Given that obesity is an established independent risk factor for psoriasis, and that weight reduction can significantly alleviate disease severity, semaglutide’s robust efficacy in promoting weight loss provides an additional therapeutic rationale.91
In a case study involving a 73-year-old obese male patient with severe plaque psoriasis (PASI score, 33.2), previously resistant to topical treatments and biologics (adalimumab), weekly subcutaneous semaglutide injections (dose escalation from 0.25 mg/week to 1 mg/week) achieved remarkable psoriasis improvement.87 After 4 months, the patient’s PASI score decreased by 76.0%, improving further to 92.2% at 10 months, alongside normalized glycemic parameters and significant weight reduction.87 The treatment was notably well-tolerated, highlighting semaglutide’s potential as an effective, cost-efficient, multitarget therapy for patients with psoriasis and concomitant type 2 diabetes and obesity.87 However, broader clinical validation through clinical studies is necessary before widespread adoption.
Conclusions
When choosing a treatment for psoriasis several factors should be considered: the location, size, and severity of lesions; the response to prior therapies; the impact of those therapies on a patient’s quality of life; long-term efficacy of current treatment options; and contraindications for individual patients. Topical corticosteroids are highly effective for localized disease but can cause skin atrophy, striae, and systemic adverse effects with prolonged use. Vitamin D analogs and calcineurin inhibitors are safer for long-term use but may cause irritation and burning sensations. Systemic therapies, including methotrexate, cyclosporine, and acitretin, are effective for moderate to severe psoriasis but carry risks of hepatotoxicity, nephrotoxicity, and teratogenicity, respectively. Biologics such as TNF-α inhibitors, IL-12/23 inhibitors, and especially the IL-17 inhibitors have shown superior long-term efficacy with favorable safety profiles, although they may increase the risk of infections and malignancies. Phototherapy is commonly used as an adjunct to topical therapies but poses some risk of malignancy and tends to be impractical for most patients.
The emerging therapies discussed in this review may offer benefits to patients for whom traditional therapies are contraindicated or for those who are opposed to traditional management. These therapies can also improve the effect of topical therapies while minimizing risk. Noninvasive therapies, such as cryotherapy, can benefit patients with localized mild to moderate disease, especially those with palmoplantar psoriasis. Microneedles and iontophoresis show promise in improving the absorption of topical therapies at a lower dose. Bland electrotherapy seems to modulate the cells and cytokines that are central to the pathogenesis of psoriasis. Finally, emerging drug repurposing strategies, such as RIPK1 inhibition with primidone and GLP-1 receptor agonism with semaglutide, have shown early promise in reducing PASI scores and inflammatory markers in preclinical and case-based studies, heralding a novel adjunctive approach for patients with refractory or comorbid psoriasis. Nonetheless, future research should focus on large-scale clinical trials to assess the efficacy, safety, and long-term outcomes of these technologies, as well as standardized protocols for their use in diverse patient populations. Such studies should also address practical considerations, such as ease of use, patient adherence, and access.
Conflict of Interest Statement
None reported.