Introduction
The use of direct oral anticoagulants has continued to increase since the approval of the direct thrombin inhibitor, dabigatran, in 2010. This was followed by the ratification of direct factor Xa inhibitors, which included rivaroxaban, apixaban, and edoxaban, within a 5-year time span.1 These oral anticoagulants provided efficacious alternatives over traditional warfarin due to their comparative safety profile, ease of use, and practical advantage of not requiring routine coagulation monitoring. Administration indications include stroke prevention in patients with nonvalvular atrial fibrillation, as well as secondary prevention of deep-vein thrombosis and pulmonary embolism.2 Although this newer class of drugs represents an important advancement in anticoagulation therapy and is touted as revolutionary, concern over the lack of reversal agents has hampered enthusiasm for its use. This is in large part because more than 84,000 patients taking direct factor Xa inhibitors are hospitalized every year in the United States due to major bleeds.3 After the bleeding begins, the risk of death or disability can be high. The 30-day mortality rate is 15% to 20% for patients who develop a major bleed while anticoagulated on these medications.4 Fortunately, the entry of andexanet alfa (Andexxa) to the market in May 2018 may prove groundbreaking; this is the first known reversal agent for a subset of direct factor Xa inhibitors.2
Andexxa is a recombinant decoy protein that rapidly reverses the anticoagulant effects of 2 direct oral anticoagulants, apixaban and rivaroxaban.2 It is specifically approved by the US Food and Drug Administration to provide acute reversal of these 2 agents in life-threatening situations or other acute uncontrolled bleeding events. Two randomized phase 3 trials have already been conducted with the therapy and have demonstrated a more than 90% decrease of factor Xa activity from baseline levels within 2 to 5 minutes. The 2 most common reasons for administration are intracranial hemorrhages (ICHs) and gastrointestinal bleeds (GIBs), and study results in these populations have been promising. For instance, when the drug has been administered to patients with ICH, mortality has been found to decrease from a baseline of 35% to 48% to a reduced rate of 12%.4,5Additionally, ongoing studies have demonstrated a limited adverse effect profile, with reported adverse effects primarily consisting of infusion-related reactions, such as flushing.6
Given the relative newness of Andexxa, it is not currently a part of most hospital formularies nor incorporated into all medical guidelines. Instead, the current medical standards of care state that when a patient develops a bleed while taking a factor Xa inhibitor, supportive interventions should begin; these often include administration of a prothrombin complex concentrate (PCC) and/or activated PCC (aPCC) until bleeding is controlled.7 Although the average hospital costs associated with non-Andexxa interventions during an ICH or GIB are between $30,000 and $40,000, these methods are not very therapeutically effective.8 Conversely, 1 dose of Andexxa will typically cost between $24,750 and $49,500 alone; it does not need to be administered alongside PCC or aPCC.6,9 While data suggest that Andexxa may be highly efficacious from a therapeutic perspective, little is known about its cost-effectiveness from an economic perspective. Major questions exist over whether this medication should be kept in hospital formularies or be offered to patients given that average hospitalization costs may substantially increase if this drug is administered.
Our study aimed to use a cost-effectiveness analysis (CEA) to address whether it is cost-effective to administer Andexxa or to follow the current standard of care for patients who develop life-threatening and uncontrolled bleeds secondary to apixaban and rivaroxaban. A CEA was used as opposed to a cost-benefit analysis because the intangible costs associated with such bleeds, such as human suffering, are not easily accounted for and would likely produce inconclusive results. Additionally, usage of a CEA allowed us to determine our main outcome goals: (1) cost and (2) quality-adjusted life-years (QALYs). Often used in economic evaluation, a QALY is a generic measure used to assess the burden of disease on both the quality and quantity of life lived. A societal perspective was also used in this CEA. This is a widely used technique of applied welfare economics to help make optimal decisions for society as a whole.10 Given that the research for Andexxa is currently incomplete but ongoing, our analysis not only modeled the current cost-effectiveness of Andexxa, but also used sensitivity analyses to illustrate the improvement in a patient’s QALYs that is necessary to consider Andexxa to be cost-effective in incidences of GIB or ICH.
Methods
Perspective
The societal perspective encompasses the major costs associated for patients with atrial fibrillation taking apixaban or rivaroxaban with the onset of an acute GIB or ICH. These costs consist of the acute factor Xa inhibitor-reversal treatment regimen, the necessary after care (including posthospitalization care), and account for the severity of the adverse event. Patients are followed from age 65 years until death or for 35 years after the onset of the adverse event. Using a societal perspective can help determine whether the use of Andexxa is financially beneficial when looking at long-term costs. A more comprehensive account of the models used, and costs incorporated, to perform the CEA are detailed here.
Model
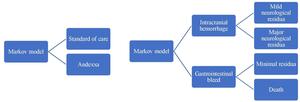
Our team designed a Markov model to compare 2 treatment strategies for the care of ICH and GIB in patients taking apixaban or rivaroxaban. The 2 modes of treatment we assessed included the current standard of care using combined infusion of Feiba (a type of aPCC) and Kcentra (a type of PCC) vs the administration of Andexxa at the time of the bleeding event diagnosis.
The starting population in this model was a hypothetical cohort of patients 65 years of age at increased risk of stroke due to nonvalvular atrial fibrillation and with no contraindication to anticoagulation. We chose this population because these patients are 1 of the largest consumers of direct factor Xa inhibitors. There have also been multiple large-scale studies done on this population; using these patients as our base population significantly reduced the number of unknown variables in our study.11 Next, these patients were diagnosed as having an acute or life-threatening major bleeding event (ICH or GIB) while taking apixaban or rivaroxaban. After the onset of the major bleeding event, it would be assumed that patients will either resume, stop, or change their antithrombotic regimen depending on their current state of health, the type of bleeding event that occurred, or their personal preference. However, given the variability in medication treatment after the bleeding event, driven partially by differing medical conditions and patient preferences, the costs of taking antithrombotic agents after the major bleeding event were not included in the cost-effectiveness model.7,12
We derived base-case assumptions for the rest of the model primarily from Andexxa trial data and medical literature. However, because there is insufficient data regarding the impact of Andexxa on GIB mortality, our research team used a value of 5% for the purposes of our base calculations. We chose this value because medical literature states that the mortality rate associated with a GIB while taking a direct factor Xa inhibitor is approximately 10.2%.13 Furthermore, intermittent ANNEXA-4 trial data suggest that mortality rates in patients with ICH are reduced by 60% when treated with Andexxa vs the current standard of care. Moreover, in patients with GIB in particular, Andexxa use has shown promising results. According to Kaatz et al, 14 interim trial data suggest that excellent or good hemostasis was achieved in 84% (95% CI, 64%-96%) of cases with GIB. This value indicates a 20% or less decrease in hemoglobin and hematocrit levels at 12 hours. Thus, for the purposes of our CEA, we believe a realistic estimate is that Andexxa treatment for GIB complicated by rivaroxaban or apixaban can decrease mortality rates from 10.2% to 5.0% compared with the current standard of care. This represents a 51% reduction in the mortality rate, similar to that observed in patients with ICH.
Next, our model applied the relative risk of death for patients’ particular states of health. Patients were followed for 35 years and mortality rates were adjusted for age and health status. The health states used in this model were mutually exclusive and the transition between health states occurred after the major adverse bleeding event. Health states associated with ICH are neurological events with minor residua, neurological events with major residua, and death. In this model, it was further assumed that patients’ life spans did not differ if they survived an ICH and sustained either major or minor neurological residua; however, it is assumed that costs and QALYs do substantially differ between these patient groups given the higher degree of disability patients with major residua will likely experience. For GIB, the associated health states were minimal residua and death. Further discussion of QALYs and health states are in the next section. A gross overview of the model is depicted in Figure 1.
QALYs
Calculating QALYs requires data that express health-related quality of life (HRQOL) in the form of a single value that is known as a health state, a utility value that is scored on a scale from 0 to 1. A value of 1 equates to a year of perfect health, a value between 0 and 1 represents a year with an intermediate state of health, and a value of 0 indicates death. These utility values were then multiplied by the time spent in each corresponding health state to calculate the QALYs.10 The health state utility values used in this study were estimates derived from population-specific medical literature. The baseline patient utility value was adjusted for disease state and the value derived from medical literature was 0.81.15 Once an adverse event, such as a GIB or an ICH, occurred in a patient taking apixaban or rivaroxaban, utility values were adjusted in accordance with the health status states depicted in the Markov model.
For patients in the Markov model treatment arm who received Andexxa, assumptions based on ANNEXA-4 trial data were used to assign health utility values for ICH.16 In our model, we specifically allotted a permanent decrement in health utility value consistent with a neurological event with minor residua for patients who sustained a nonfatal ICH and received Andexxa. We chose this value because of the promising data stemming from ANNEXA-4 interim trial results. For instance, for patients who receive Andexxa following a nonfatal ICH, excellent or good hemostasis has been achieved in 80% (95% CI, 56%-94%) of cases. These values indicate 35% or less increase in hemoglobin and hematocrit levels at 12 hours.14 From these data, we assumed that patients who received Andexxa for ICH may have milder neurological residua, which is why we assigned them a health utility value consistent with mild neurological residua. For patients with GIB, we used the same health utility value for them regardless of whether they received Andexxa or non-Andexxa intervention. This is because a minimal decrement in quality of life is expected for patients with GIB long-term.17
Costs
In this CEA, we primarily examined the direct major costs associated with medical interventions. Microcosts were not directly examined due to difficulty in capturing all relevant costs, but many microcosts are indirectly accounted for in QALY calculations. Moreover, costs associated with lost productivity were not accounted for given that the base population for this analysis was initially 65 years old, the age at which many individuals have retired from the workforce. For most associated costs, utilities, and ratios used in the analysis, see Table 1.
Macrocosts for the analysis regarding major 1-time events were derived from the literature and primarily estimated from 2014 mean costs published online by the Agency for Healthcare Research and Quality from the Healthcare Cost and Utilization Project data under relevant primary International Classification of Diseases, Ninth Revision codes.8 Mean prescription drug costs were derived from various hospitals’ published formulary costs and pharmaceutical companies.6,17–21 Costs and QALYs were then calculated in each cycle according to the health state the patient occupied. The costs accrued for each Markov state were weighted according to the amount of time a person spent in each health state. Summations were calculated for each treatment and a discount rate of 3% per year was applied.10 Relevant long-term costs were derived from medical literature and the overall analysis was modeled over 35 years.17 Of note, as GIBs do not typically incur significant long-term rehabilitation or care costs, no cost was accounted for them in the model.5
Furthermore, the macrocosts associated with Andexxa’s adverse effects were not included in this analysis due to ongoing data collection in the ANNEXA-4 trial. Known adverse effects associated with the standard of care cannot be modeled in comparison with the adverse effects associated with Andexxa either. This is because clinical trials are conducted under widely varying conditions; hence, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared with the rates in the clinical trials of another drug. Moreover, they may not reflect the rates observed in clinical practice.6
Sensitivity Analyses
When conducting CEAs, there are multiple uncertainties that consist of the parameter of inputs chosen, the associated interventions, and the fit of the model itself. Performing 1-way and 2-way sensitivity analyses were useful as they allowed us to better quantify the impact of each individual parameter within our model and to assess whether our model was a good fit. These analyses can be particularly useful for new interventions where there is still significant uncertainty. Moreover, sensitivity analyses enable the calculation of the relative clinical benefit of Andexxa using incremental cost-effectiveness ratios (ICERs).10 ICERs illustrated the cost per QALY gained for Andexxa compared with the standard of care. Use of these ratios can help assess whether the benefit accrued with Andexxa utilization was within the willingness-to-pay (WTP) cost threshold per added QALY. WTP is the maximum price range at or below which policymakers should consider offering Andexxa. Because there are different WTP thresholds nationwide and across the globe, our sensitivity analyses reflect a range of input variables and ICERs to account for a US WTP range of $50,000 to $150,000 per QALY.22,23 Hence, to better understand the relationships between input and output variables in our model, we used 1-way and 2-way sensitivity analyses.
Results
Base-Case Analysis
Intracranial Hemorrhage. Under our base-case assumptions for patients who sustain an ICH while taking apixaban or rivaroxaban, we calculated total QALYs of 1.03 for the current standard of care and 1.38 with the use of Andexxa. With current standard of care, the net present value of total major costs was $170,277.09. For Andexxa, this value was $242,656.20. Utilization of Andexxa thus resulted in a gain of 0.35 QALYs at an incremental cost of $72,433. The ICER for use of Andexxa vs current standard of care for reversal of apixaban or rivaroxaban associated ICH was $211,056. Under these base-case assumptions, in cost-effectiveness terms, no one mode of treatment is economically dominant to the other.
Gastrointestinal Bleeding. Under our base-case assumptions for patients who sustain a GIB while taking apixaban or rivaroxaban, the calculated total QALYs was 9.37 for the current standard of care and 9.89 with the use of Andexxa. The net present value of total major costs with current standard of care was $9,730 and $30,928 with Andexxa. Utilization of Andexxa resulted in a gain of 0.52 QALYs at an incremental cost of $21,198. The ICER for use of Andexxa vs current standard of care for reversal of apixaban- or rivaroxaban-associated GIB was $40,718. Under the base-case assumptions, in cost-effectiveness terms, no one medical intervention was dominant to the other.
Sensitivity Analyses
Overview. The sensitivity analyses showed that the factors with the most influence on the cost-effectiveness of Andexxa included the cost of the medication, mortality rates, relative risks of death, and HRQOL after Andexxa administration.
Medication Cost. The medication cost sensitivity analysis for Andexxa showed how the ICER for Andexxa decreases as the medication cost decreases (Figure 2). Currently, most patients will require 1 dose of Andexxa, which will generally cost between $25,000 and $31,000 for the medication alone.6 For GIB, the ICER for Andexxa satisfies the US WTP threshold at the current cost of the medication. However, from an ICH perspective, the sensitivity analysis clearly delineated that the ICER for Andexxa exceeds the US WTP threshold at the current price of Andexxa. Even if the cost of the medication decreases by half, to approximately $15,000 total per patient, the ICER for Andexxa in incidences of ICH would still be $164,487; this ratio exceeds WTP thresholds per QALY in the United States and other industrialized nations. Figure 2 illustrates this sensitivity analysis.
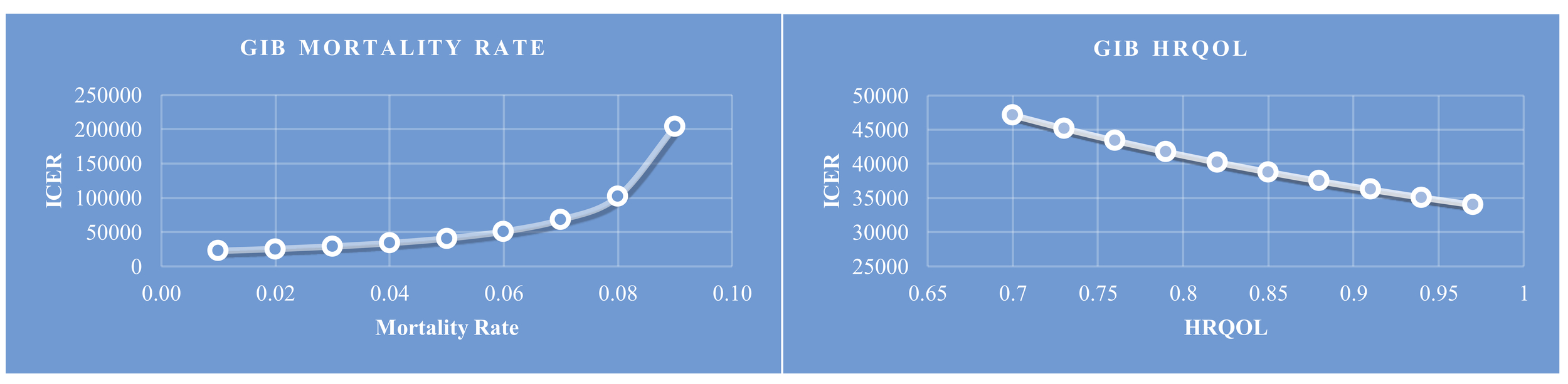
Gastrointestinal Bleed. From solely a GIB perspective, if Andexxa use decreases the mortality rate by approximately 50% (from 10.2% to 5%) for GIB, then the ICER for Andexxa in GIB would be approximately $41,000; this would satisfy the lowest range of the US WTP threshold (Figure 3A). However, given the uncertainty of the effect of Andexxa on GIB mortality rates, a sensitivity analysis can better model the effect on mortality rates that Andexxa would need to have to be cost-effective.6 This analysis showed that an ICER of $101,795 would be achieved if a 21.6% decrease in GIB mortality rate occurred with Andexxa use (mortality rate decreases from 10.2% to 8.0%); such a decrease in GIB mortality would allow Andexxa to satisfy a WTP range between $100,000 and $150,000. The sensitivity analyses further illustrated the effect Andexxa would need to have on HRQOL to be cost-effective (Figure 3B). From medical literature, we currently know that the decrement associated with QALY for a GIB is approximately 0.05.17 Moreover, the ICER for Andexxa would continue to satisfy our lowest WTP threshold of $50,000 if patients’ health utility values generally remained above 0.7 following Andexxa administration. These analyses are depicted in Figure 3.
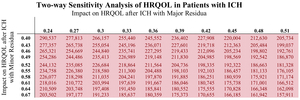
Intracranial Hemorrhage. In comparison with use of Andexxa during GIB, the use of Andexxa during ICHs is not as cost-effective. The sensitivity analyses showed that the largest drivers of cost-effectiveness for patients with ICH following Andexxa treatment are patient mortality rates (Table 2), the rates of severe neurological residua (Table 2), the impact of major and minor neurological residua on HRQOL (Table 3), and the relative risk of death.
After reviewing available Andexxa data, we made our base-case assumptions such that the initial chance of death was 0.14 following Andexxa for ICH and the initial chance at major neurological residua was 0.55.4–6 This resulted in a calculated ICER of approximately $211,056. If either of these assumptions were discovered to be incorrect once more data are released from the ANNEXA-4 trial, Table 2 shows what these 2 variables may need to be for Andexxa to meet different WTP thresholds. Overall, for Andexxa to be cost-effective in ICH, it would need to significantly lower patient mortality rates and lower rates of patients developing major neurological residua following treatment.
Another major factor of Andexxa that is currently unknown is its impact on patients’ HRQOL following treatment. Current literature suggests that in patients with ICH and atrial fibrillation who receive the standard of care following an ICH with minor and major residua, their health utility states drop to 0.52 and 0.45 from baseline, respectively.17 If we assume all patients’ health states drop to a utility value of 0.52 with Andexxa use as is assumed under our base-case assumptions, the ICER is approximately $211,056 following Andexxa administration. However, we suspect that patients’ levels of disability would not be the same following Andexxa administration vs the standard of care given how Andexxa rapid reverses direct factor Xa inhibition. Thus, Table 3 models what the effects of Andexxa on HRQOL following an ICH would need to be to meet the standard WTP range of $50,000 to $150,000.
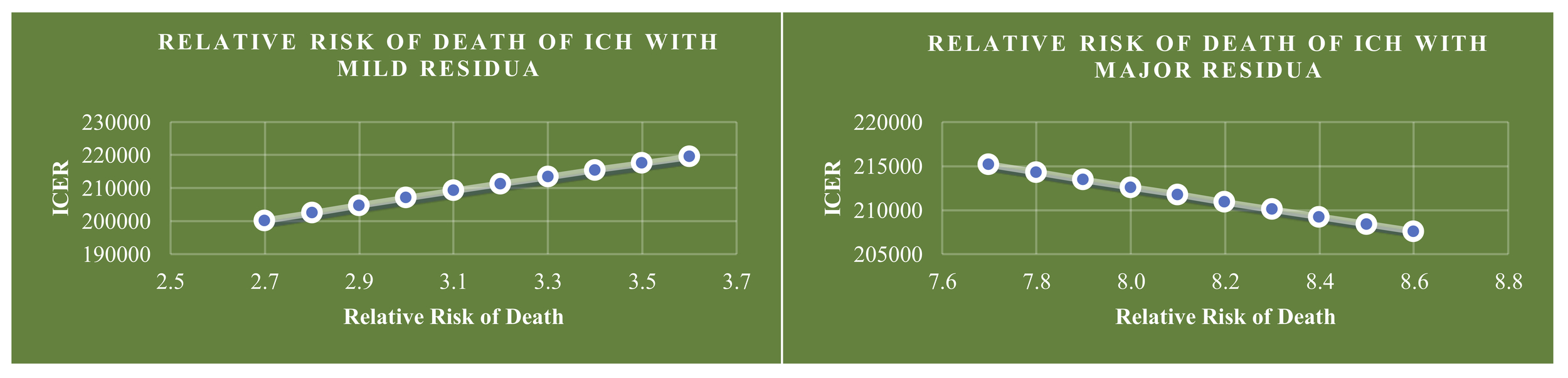
Finally, our current base-case model assumed that a patient’s relative risk of death following an ICH with minor neurologic residua was 3.18 and a patient’s relative risk of death following an ICH with major neurologic residua was 8.2 (Table 1). To be cost-effective, Andexxa would need to substantially reduce the relative risk of death following an ICH in incidences of minor neurological residua. In incidences where a patient is highly susceptible to developing major neurological residua following an ICH, administration of Andexxa would not be cost-effective. This sensitivity analysis is illustrated in further detail in Figure 4.
Discussion
Review of the results indicated that the current cost-effectiveness of Andexxa depends significantly on the condition for which Andexxa is being administered, the effect of Andexxa on the patient’s disease state and mortality, and from who’s perspective—the payer or the patient— the utility of Andexxa is being assessed. Currently known data demonstrate that Andexxa rapidly reverses the anticoagulant effect of certain direct factor Xa inhibitors in patients with major bleeds, but more data are needed to better assess the cost-effectiveness and utility of Andexxa for various disease states. Studying Andexxa solely from a cost-effectiveness perspective determines that society would benefit from hospitals and insurance companies carrying and reimbursing this medication for GIB. However, on analysis of currently known data, using Andexxa to treat ICH complicated by concurrent direct factor Xa inhibitors may be above most societies’ WTP thresholds.
When looking beyond the scope of a CEA in making health policy decisions, multiple ethical issues arise. If observing Andexxa from a patient’s or a caregiver’s standpoint, administering the most effective medical regime, independent of the costs, seems like a reasonable expectation. Similar dilemmas often arise in health care on the introduction of new medications, diagnostic tests, or interventions. If patients’ health improve or lives are saved with Andexxa therapy, the public could potentially have a strong reaction if best practice is not used.
A second potential ethical issue concerns the diagnosis-related group (DRG) payment system. Hospitals may be tentative about carrying and administering Andexxa given the increase in the cost, especially if there are not increases in the DRG payment that coincide. In a fee-for-service system, insurance companies may also be reluctant to cover Andexxa. The cost trend can also be worrisome for patients as they may be forced to bear a bigger share of their medical expenses in the form of an increased out-of-pocket expense. In the next trial phase, if Andexxa proves to be beneficial, health care leaders could look at potential solutions to mitigate the cost of this drug. This could include evaluating current patent protections and discussing the degree of monetary investment that should go into the future development and distribution of this medication.
As discussed here, there are multiple limitations to our CEA due to missing data as the ANNEXA-4 trial is still ongoing. For instance, there are insufficient data regarding the adverse effects of Andexxa in a practical setting and the potential costs associated with treating said adverse effects. Estimates for unknown data like these could not be included in our model. Additionally, due to practicality, microcosts were not directly captured in our survey, but may indirectly be accounted for in other QALY calculations due to changes in patients’ health states. Moreover, multiple key assumptions were included in our model. One such assumption was our estimates of mortality rates associated with both ICH and GIB after utilization of Andexxa; true mortality rates are unknown. Furthermore, do not know the effect that Andexxa will have on health utility states and the impact it will have on long-term costs associated with a patient’s postacute care, such as rehabilitation costs. Assumptions were made regarding these values based on relevant literature. Finally, another important limitation was that our analysis looked at Andexxa viability for older adults who had atrial fibrillation. Reasons for this were described in the Methods section. In the clinical world, however, patients who could potentially benefit from Andexxa span across all age groups that use anticoagulants for various medical conditions. Given that our analysis did not account for all the potential patients who could benefit from the medication, conducting repeat CEAs for different patient groups with this missing information will be crucial to understanding the cost-effectiveness of the drug.
By May 2019, Andexxa will have been on the market for a complete year and the latest information gained from the ANNEXA-4 trial will be critical towards understanding the drug’s associated adverse effects and health outcomes. In the future, further analysis should be done to better understand the associated health costs for people who were administered Andexxa during a major bleed. Once the effects of Andexxa are studied on various patient groups and missing variables are filled, analysis can be redone to understand whether Andexxa is cost-effective for the general population. Analysis can also be done on the data for finding out for which participants Andexxa would be most cost-effective. If these data are promising from both a medical and cost-effectiveness vantage point, policy changes should be implemented to require hospitals to carry Andexxa, to encourage insurance companies to reimburse the intervention, and to account for its value under the DRG-based payment system. If data in the future are promising from a medical vantage point solely, and not from a cost-effectiveness perspective, then insurance companies and hospitals should strongly consider carrying Andexxa from an ethical standpoint.
Acknowledgement
David Hutton, PhD, Associate Professor in the Department of Health Management and Policy and Department of Industrial Operations Engineering, University of Michigan, Ann Arbor, Michigan, provided extensive research design and editorial assistance.
Disclosures
There are no disclosures to report, no funding sources to report, and no conflicts of interest for any of the authors.