Introduction
Glioblastoma multiforme (GBM) is both the most common and most aggressive primary central nervous system (CNS) malignancy. It is a World Health Organization grade IV tumor of astrocytic lineage that remains incurable to date.1 GBM accounts for 45.2% of primary CNS malignancies and 16% of all CNS tumors, and has a median survival of 12 to 15 months.1,2 A hallmark of malignant gliomas, including GBM, is that at the time of clinical presentation, the tumor is already widely disseminated throughout the CNS.3 These invasive micrometastases throughout the brain parenchyma preclude the possibility of a curative surgical resection and nearly assure tumor recurrences.3 Standard care treatments, including surgical resection, radiotherapy, and chemotherapy, have a limited effect on survival, despite this multimodal approach to therapy; less than 10% of patients survive beyond 2 years.2 Thus, there is an urgent need for continued research efforts for more efficacious therapeutics. Oncolytic virotherapy (OV) uses competent viruses in a 2-pronged approach to first directly infect and lyse cells, while acting as an immunogenic agent to stimulate an antiviral immunotherapeutic response.4 Several pathogenic viruses have been extensively explored for oncolytic viral therapy such as herpes simplex virus, adenovirus, and, poliovirus, which is the topic in this review.2 The unifying principles underlying each of these are conditional replication, cytoxicity in cancerous cells, and reduced propagation in normal tissues.5
Poliovirus Sabin rhinovirus IRES poliovirus open reading frame PVS(RIPO) is a poliovirus-rhinovirus chimera engineered from a live-attenuated poliovirus type 1 (Sabin) virus that is currently under clinical investigation for GBM. Here, I review the rationale for its use, development, mechanisms of action, preclinical experiments, ongoing clinical trial, and limitations in GBM therapy.
Introduction to Oncolytic Virotherapy and Poliovirus
OV is developing as a promising therapeutic strategy in the treatment of several malignancies, including GBM. While it was first observed approximately a century ago that viral infections had the capacity to fight cancers, viral agents have re-emerged at the forefront of developing cancer therapies due to improved genetic understanding and manipulation.5 This has led to increased tumor-targeting specificity and decreased off-target toxicity of oncolytic viral agents.6 Several pathogenic viruses have since undergone investigation, such as herpes simplex virus type 1, the first to be considered for glioma oncolysis; adenovirus, the first oncolytic virus that went under clinical investigation; and poliovirus, which has shown significant therapeutic potential for prolonged survival and mortality benefit in patients with GBM.6
Poliovirus is an enterovirus in the Picornaviridae family of viruses and is the causative agent in paralytic poliomyelitis via selective destruction of motor neurons.7,8 Therefore, the inherent neuropathogenicity of wild-type poliovirus precludes it from therapeutic use. Genetically recombinant variations, however, are now being recognized as possible therapies in the treatment of solid malignancies, including GBM.9 This requires diverting the pathogenicity of poliovirus towards a therapeutic effect by limiting replication in neuronal tissue, while allowing replication in nonneuronal tissue.10 Such viruses have stringent requirements: viruses must (1) be nonpathogenic, (2) be genetically stable on intratumoral replication, (3) target and infect notoriously heterogeneous tumors, (4) elicit efficient killing of infected tumor cells, (5) act in the presence of neutralizing antibodies and innate antiviral immune activation, (6) reverse the immune suppressive microenvironment, and (7) be capable of recruiting immune effector arms against the tumor target (Figure 1).10 Combining these necessary features into a single agent is a significant challenge and required extensive preclinical research, as summarized in Figure 1.
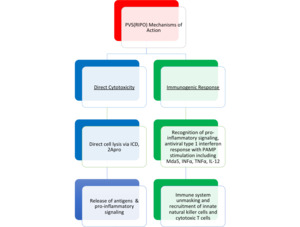
Oncolytic viruses demonstrate therapeutic responses against cancer through a 2-pronged approach with functionally intertwined mechanisms, which are summarized in Figure 2. The first mechanism is direct cytotoxicity and the second is the subsequent immunogenic response. OV may directly infect and lyse cancer cells (first prong), while simultaneously activating inflammatory antiviral pathways (second prong).11 Infection and subsequent tumor cell lysis is a means of propagation as the process releases viral progeny that can spread and infect surrounding malignant cells with the appropriate receptors. Throughout this process, cancer cells infected with virus can present viral proteins through the major histocompatibility complex, which can be recognized by the cell-mediated immune system and marked for destruction by cytotoxic T lymphocytes (second prong).3,12 Essential to effective therapy is cancer cell–specific targeting, while preserving normal host tissue. Numerous strategies have been used to achieve such specificity including altering viral tropism, deleting viral virulence genes, or placing viral genes under the control of tumor-specific promoters.13
Rationale for Oncolytic Poliovirus and Necl-5 Receptor
For OV to be effective, it must have tropism for cancerous cells, while preserving normal host tissue. Tropism, the single most important factor in the efficacy of OV, is defined as the ability of a virus to recognize cell surface features that mediate viral genome entry into the host cell. 10 The promising nature of PVS(RIPO) is due, in part, to fact that the poliovirus receptor nectin-like molecule 5 (Necl-5, also called cluster of differentiation 155) is broadly ectopically upregulated in GBM (along with many other types of solid neoplasms).6,7,14,15 Poliovirus tropism uniquely depends on Necl-5 because all attachment and entry events for poliovirus are mediated by this single molecule.16 Additionally, Necl-5 is expressed on antigen-presenting cells and this is of particular importance for GBM, in which macrophages and myeloid-derived suppressor cells comprise a significant portion of tumors.4 Infection with both poliovirus and PVS(RIPO) demonstrate that infected antigen presenting cells (APCs) facilitate antigen presentation and immune effector function, which may contribute to antitumor efficacy.10 Necl-5 is a regulator of cancer invasiveness, migration, contact inhibition, cancer cell motility, and NK cell activity, and it can become transcriptionally upregulated during DNA damage signaling.4,15 On binding of the poliovirus to Necl-5, the poliovirus receptor catalyzes an irreversible expansion resulting in the externalization of the myristoylated capsid protein VP4 and the N-terminal extension of the capsid protein VP1 on the cell membrane.17 These mechanisms may relate the disintegrating viral capsid with the transfer of the viral RNA genome.4 Once the viral RNA genome is present in the cytoplasm, it is immediately translated, initiating the subsequent steps in oncolysis. This is the critical rate-limiting step as establishing an infection depends on the immediate translation of the incoming viral RNA and failure to translate will abort the infection process.10
Development of PVS(RIPO)
As mentioned previously, the pathogenicity of wild-type poliovirus in neuronal cells precludes it from therapeutic use. A chimeric virus composed of the poliovirus and rhinovirus was made to attenuate viral reproduction and neurovirulence in neuronal cells, while allowing conditional replication in nonneuronal tissue. To remove the inherent neuropathogenicity, the internal ribosomal entry site (IRES) of the PV1 Sabin vaccine strain was genetically manipulated.18 IRES is a highly structured cis-acting genetic element of approximately 500 nucleotide length in the poliovirus 5’ untranslated region that is essential for driving viral translation initiation. The poliovirus IRES was replaced with the IRES of the human rhinovirus 2 (HRV2), leading to the elimination of neurovirulence, conditional replication, and cytoxicity in cancerous glial cells, while reducing propagation potential in cells of neuronal derivation.5,18 Neurovirulence was determined to rely on domains V and VI within the poliovirus IRES and when it was removed, the neurovirulence was also lost.18
More specifically, the loss of neurovirulence was due to alteration in the mechanism of protein synthesis. Poliovirus RNA is uncapped and must, therefore, use a nonconventional translation mechanism, which is defined by the direct recruitment of the eIF4F:4A:4B translation complex, which has helicase functionality.10,19 The IRES genetic element enables the binding of the eIF4G:4A:4B complex and is the rate-limiting step in the initial translation of poliovirus.10 By substituting in the HRV2 IRES, the ability of the recombinant poliovirus to recruit the helicase translation complex was eliminated in normal CNS cells.10,20 The growth deficits induced by the IRES substitution are likely caused by several factors. Two proposed causes of the interference in translation are: (1) differences in cell type–specific recruitment of preinitiation translational complexes and (2) differences in interaction between host neuronal cell RNA-binding proteins (DRBP76) and the viral HRV2 IRES.10,21
This IRES substitution is the source of the genetic stability of recombinant virus compared with previously generated vaccines, which contained point mutations.10 This recombinant poliovirus is the prototype oncolytic polio/rhinovirus termed PVS(RIPO).4 It is the live-attenuated poliovirus type 1 (Sabin) that contains a heterologous IRES derived from HRV2.5
Attenuated Neurovirulence and Conditional Replication
A mechanism of the attenuation of neurovirulence was reported in 2006 that describes conditional replication of the HRV2-containing PVS(RIPO) in nonneuronal cells.20,22 Merrill et al20,22 proposed a mechanism whereby a set of 5 proteins found in the cytosol of normal neuronal cells regulates and limits the replication and propagation of PVS(RIPO). The most notably regulatory protein in this series is double-stranded RNA-binding protein (DRBP76). The mechanism of DRBP76 regulation to limit viral replication is via posttranscriptional modification of mRNA in neuronal cells that specifically depends on the HRV2 IRES element in PVS(RIPO). The posttranscriptional modification of mRNA eliminates viral replication and propagation in neuronal cells.20 Regulation of mRNA thereby decreases global rates of protein synthesis in the cell and, thus, selectively abrogated neuropathogenicity in neuronal cells.
It was found that the regulatory protein DRBP76 in the cytosol of normal neuronal cells selectively associates with HRV2 and binds viral mRNA in neuronal cells but not in malignant glioma cells.20,22 This could be due to the localization of DRBP76 in the nuclear compartment in neoplastic cells, while normal neuronal cells express DRBP76 in the cytoplasm.4
The group also found that when DRBP76 was depleted in a neuronal cell line, viral replication and propagation were enhanced.20 While the underlying mechanisms have yet to be elucidated, these results demonstrate the importance of the microenvironment and viral specificity in the intracellular milieu.
Translational Regulation of PVS(RIPO)
As previously mentioned, poliovirus mRNA uses an unconventional mechanism of translation: the cap-independent mechanism. In neoplastic cells, viral translation and proliferation are enhanced due to relaxed conditions for translation initiation due to broadly deregulated mitogenic signal transduction cascades favoring cap-independent translation.4 This is of particular relevance because GBM is frequently associated with alterations of genes encoding proteins involved in mitogenic signal transduction such as widespread activation of Ras/MAPK signaling.21,23
In 2010, it was reported that growth and cytotoxicity of PVS(RIPO) in GBM is promoted by Ras/MAPK (mitogen-activated protein kinase) signaling and PI3K inhibition.21 While the detailed mechanism is beyond the scope of this review, it was found that viral translation of PVS(RIPO) was facilitated by the Ras/MAPK pathway and the MAPK substrate Mnk1 (MAPK signal integrating kinase 1).21 MAPK and Mnk1 were found to suppress serine and arginine protein kinases (SRPKs). Decreased SRPK activity enhanced IRES-mediated viral translation of PVS(RIPO) and cytotoxicity as SRPK is hypothesized to regulate IRES competency, cytotoxicity, and viral proliferation.23,24
The group performed interesting in vitro and in vivo studies using GBM xenografts that demonstrated increased cytotoxicity and enhanced tumor regression when PVS(RIPO) was administered with PI3K inhibitors.21 In 2010, Goetz et al performed a notable experiment that showed that introducing oncogenic Ras into neuron-like cell line HEK293 re-establishes translation and cytotoxicity of PVS(RIPO) in a cell line that is normally resistant to the translation and cytotoxicity of PVS(RIPO).21
In sum, suppression of SRPK plays a critical role in the promotion of viral translation. The mechanism of precisely how negative regulation of SRPK confers viral IRES-mediated translation remains under investigation. While other mechanisms are suspected to participate in translational regulation, it is clear that SRPK activity is a major determinant and regulator of IRES competency, cytotoxicity, and viral proliferation in infected cells.4
Cytotoxic Mechanism of PVS(RIPO)
PVS(RIPO) uses a 2-pronged approach to directly kill cancer cells, while simultaneously stimulating an immunologic response in the host (Figure 2). This hypothesis contrasts traditional views, which claim they are functionally distinct.10 These 2 mechanisms of action are, however, intimately linked mechanistically and biologically, as evidenced by the immunologic stimulating process of immunogenic cell death (ICD), a noncanonical form of cell death stimulated by viruses that promotes an immune response against the antigens in the dead cells.10 ICD is characterized by apoptosis with concomitant release of adenosine triphosphate, the proinflammatory cytokine high-mobility group box 1, and calreticulin.10 The immediate effects of the release of these molecules, in sum, are the recruitment of dendritic cells (an antigen-presenting cell) to the site of cell death, antigen presentation to cytotoxic T lymphocytes, and activation of gamma-delta T cells.10,25 The cumulative effect of ICD results in immediate cell death with an immunogenic antitumor response. This mechanism of cell death is an illustration of the intertwined mechanisms of cytotoxicity and immunogenicity.
An additional mechanism of cytotoxicity is via the enzyme 2Apro, a protease that cleaves the cell’s normal protein synthesis apparatus, which can lead to cell death.26 To our knowledge, 2Apro is the first enzyme synthesized by poliovirus in infected tumor cells. Therefore, the first step in the cytotoxic mechanism of PVS(RIPO) in tumor cells is establishing translation of viral RNA, as described above. Translational initiation in tumor cells is, therefore, the rate-limiting step in the cytotoxicity mechanism. This produces a series of very rapid, irreversible, and lethal alterations in host tumor cells. 2Apro is released as early as 60 minutes after infection of tumor cells, and it has been shown that 2Apro alone is sufficient to trigger cell death.10,27,28 2Apro can then intercept the host cell’s normal gene expression mechanism and shut off the host cell’s normal protein synthesis by degrading eukaryotic-initiating factor 4G and nuclear pore proteins.10,29 As previously mentioned, PVS(RIPO) uses an alternate cap-independent translation mechanism, PVS(RIPO), so its translational mechanism is unaffected by 2Apro activity. Therefore, the virus has subverted normal cellular function and is then able to produce viral progeny until that cell’s eventual death.
Immunogenic Mechanisms of PVS(RIPO)
The robust antitumor response generated by PVS(RIPO) is hypothesized to be mostly conferred by the immunogenic response, despite the precise immunologic mechanisms remaining unclear. The recognition of the tumor from its immunosuppressive environment is thought to be the pivotal step in the efficacy of the immune system’s antitumor response and is partially dependent on the previously described cytotoxic mechanisms.
Host immunogenic responses are thought to begin in significant effect with the process of ICD, as previously mentioned, which not only stimulates the release of proinflammatory cytokines (high-mobility group box 1), but also releases tumor-specific antigens that are presented: danger and pathogen-associated molecular patterns (DAMP and PAMP).10 The recognition of danger- and pathogen-associated molecular patterns elicits a vigorous proinflammatory response, innate immune response, and eventually an adaptive response with cytotoxic T lymphocytes. The immune response, however, may represent a double-edged sword because it eliminates infected tumor cells and it blocks viral replication and spread to remaining tumor cells.26
Cell death via ICD also activates melanoma-derived antigen 5, a cytosolic pathogen recognition receptor capable of stimulating a series of cytokines such as interferon-α, tumor necrosis factor α , and interleukin 124,30 These potent signals are also proposed to stimulate tumor-associated macrophages and dendritic and natural killer T cells, which then recruit immune effector responses directed against tumor neoantigens that arise from the cytotoxic effect of viral translation.4
In 2016, Holl et al26 examined the immune response stimulated by PVS(RIPO) in human breast and prostate cancer xenograft models in mice and found that a powerful innate inflammatory response coincided with chemokine induction and myeloid cell infiltration in tumors. In analyzing mRNA harvested from tumor xenografts, they found elevated levels of proinflammatory cytokines and activation of basophil, eosinophil, and neutrophil cells, the most commonly found infiltrating cells (CDll1+Ly6C+Ly6G+ neutrophils).26 Interestingly, CCL5 and CXCL10, 2 chemokines that were found, are also involved in T-cell recruitment, a significant contributor to the antitumor response.26 Additionally, the researchers also found significant increases in a series of enzymes used by neutrophils in cell killing such as myeloperoxidase, which makes cytotoxic free radicals, and p-Stat1, which is a downstream target of both type 1 and type 2 interferon signaling.26 While these data are derived from breast and prostate cancers, they may have implications for understanding the mechanisms of immunotherapy in malignant gliomas, such as GBM, because they highlight T-cell recruitment.
The understanding of the mechanistic link between viral cytotoxicity and antiviral immunity is fervently being investigated. The “unmasking” of the tumor to the immune system is thought to be both the critical function of oncolytic viral immunotherapy and the most enigmatic part in our understanding of immunotherapy. A greater understanding of this process will not only enhance our knowledge of the virus-host interaction, but will also broaden and inform the clinical application of oncolytic viral immunotherapy.4
Preclinical Experiments
Throughout the development process of PVS(RIPO) there have, in fact, been several iterations that have undergone extensive in vitro and in vivo experiments and have shown significant preclinical success, as summarized in Figure 1. The iterations in its development are not discussed here but, briefly, they include variations derived from the Mahoney and Sabin poliovirus strains termed PV1(RIPO), PV1(RIPOS), and the current generation PVS(RIPO).
Throughout the generations, the PV/HRV2 chimeras have continually showed overall safety with minimal neurovirulence, conditional replication, and effective cancer-cell killing. Table 1 highlights the notable preclinical in vivo experiments in rodent and nonhuman primate models that propelled PVS(RIPO) towards clinical use. Significant experiments include administering PV1(RIPO) intravenously in a mouse model at 1000 times the lethal dose 50% of wild-type poliovirus with no symptoms of clinical neurological disease.18 In 2000, Gromeier et al7 tested tumor regression with PV1(RIPO) in mouse xenograft with 2 cell lines and not only found significant tumor regression in a subcutaneous flank tumor with intratumor administration of the virus, but also synchronous tumor regression of a contralateral tumor when only the ipsilateral tumor was treated with PV1(RIPO). This has profound implications for the power of the immunogenic response and for the treatment of cancer metastasis.
A rodent study done in 2006 found both safety and tumor regression in an intracerebral rat xenograft model.31 Survival was lengthened from 23 days in the control group to 53 days in the group receiving PVS(RIPO) either intrathecally or intracerebrally.31 Furthermore, 6 animals in the treatment group survived until the end of the experiment and were followed up to 173 days.31 The study found that intrathecal or intracerebral delivery of PVS(RIPO) were both effective, and this regional delivery limited exposure of the virus to other parts of the body.31 The study concluded that PVS(RIPO) is safe and effective at the doses tested and could also be effective for intracranial metastatic tumors that express the Necl-5 receptor, highlighting the importance of tropism and the specificity of the virus.31 Notably, while the survival benefit was statistically significant, there was no significant dose-response relationship for intracerebral treatment with PVS(RIPO), meaning the higher dose did not provide a longer survival benefit. This is a feature that was similarly found in the phase 1 clinical trial.31,32
Perhaps of greatest clinical relevance for safety were the 2 experiments performed in nonhuman primate models in 1999 and 2012.33,34 The studies involved intraspinal and intrathalamic inoculations of several chimeric virus variants (PV1[RIPO], PV1[RIPOS], and PVS[RIPO]), all of which demonstrated safety and lack of neurovirulence. In 2012, extensive tests were conducted on Macaca fascicularis, a nonhuman primate; those tests include clinical tests, hematological analyses, clinical chemistry, necropsy, histopathology, biodistribution studies, shedding studies, and immunological studies.34 All efforts led to the conclusion of extraordinarily low neurovirulent potential and safety highlighted by the inability to disseminate or replicate extraneurally and lack of shedding.35 This was a critical study in support of the clinical use of PVS(RIPO) as an OV therapy for GBM.34
Phase 1 Clinical Trial
In 2011, a phase 1 clinical trial was initiated at Duke University for patients with recurrent World Health Organization grade IV GBM (NCT01491893). Patients in the study had refractory supratentorial GBM 1 to 5 cm in size; were at least 4 weeks posttreatment with chemotherapy, bevacizumab, or other study drug; had adequate organ function; scored greater than 70 on Karnofsky Performance Status Scale (classification of functional impairment); and had positive antipoliovirus titer.36
The purpose of the study is to determine the maximum tolerated dose of PVS(RIPO) when delivered intracerebrally by convection-enhanced delivery directly into the tumor microenvironment and to monitor clinical efficacy with the optimal dose. Convection-enhanced delivery involves the implantation of catheters directly into the tumor that deliver the virus with continuous, positive pressure flow.35 This drug delivery system has several benefits, such as bypassing the challenges associated with the tight junctions of the blood-brain barrier, creating high local tumor concentration of virus, and having low systemic concentrations.35 The study aims to enroll 65 patients and the determined dose would then be used in the phase 2 trial. Progression-free survival, overall survival, and radiographic response are secondary outcome measures.
As of July 2014, the trial has shown success.32 Desjardins et al32 reported 10 patients have been treated at varying dose levels from level 1, the lowest dose, to level 5, the highest dose. Several patients showed complete clinical and radiographic responses and, on May 10, 2016, the Food and Drug Administration granted Breakthrough Therapy Designation to PVS(RIPO).26 Of those 10 patients, 8 were reported alive at the date of publication, with the first 2 patients alive at 19 and 20 months after PVS(RIPO) administration, compared with an average overall survival with GBM of 12 to 15 months. The 2 patients who died 6 months after inoculation of PVS(RIPO) had previously failed treatment with bevacizumab and experienced persistent neurological deterioration.37 One unspecified dose-limiting toxicity event was reported at dose level 5, and other adverse events were reported at the lower dose levels 1 and 2 such as hemiparesis, lymphopenia, seizure, diarrhea, paresthesia, dysphasia, and hyperbilirubinemia.32 The trial was amended after observing prolonged steroid use in 5 of the 7 patients treated with the highest-level doses (levels 3-5). It was then decided that the subsequent 6 patients would be treated at dose level 2, which was the agreed on optimal dose level.37
Desjardins et al37 reported in November 2014 that 13 patients had been treated by that point and that the first 2 patients from the July report remained alive at 24 and 25 months.37 Despite the previously reported adverse events, infusion of PVS(RIPO) via convection-enhanced delivery was recognized as safe and efficacious at the level 2 dose.32,37 The trial aims to conclude in 2018, and the observed efficacy warrants further clinical investigation.
Limitations
While the preclinical and early clinical studies of PVS(RIPO) have shown significant promise for improved care, causes of concern remain. PVS(RIPO) and oncolytic virotherapy as treatments are in their infancy. The most notable concern is the fear of genetic instability after therapeutic administration. As previously mentioned, the substitution of the HRV2 IRES region is thought to provide significant stability to the virus. PVS(RIPO) was found to have significant stability in both in vitro and in vivo studies. The virus was found to have significant stability after long-term serial passaging in HeLa cells.7 Additionally, PVS(RIPO) was isolated after administration in a GBM xenograft model mouse model and underwent full-length genome sequencing, which again showed genetic stability.5 Steps have been taken to reduce genetic instability; nevertheless, the fear persists because there are no simple strategies to circumvent rapid genetic adaptation.5
A second criticism of PVS(RIPO) oncolytic therapy has been that the viral receptor that PVS(RIPO) relies on, Necl-5, is not likely to be homogeneously expressed in all tumor cells and that virus-resistant subpopulations likely do exist even with Necl-5.10 It is most likely true that not all tumor cells will contain Necl-5, but this is less concerning because the primary mechanism of OV strategies is not direct oncolytic activity but the recruitment of host immunogenic response.10 Tumor tropism is necessary to establish the infection and immunogenic response, but it is inconceivable that all malignant cells can be reached, infected, and directly eliminated even with aggressive dosing and invasive administration. While the immunogenic response is the predominate force in the antitumor response, there remains considerable room for investigation into their mechanisms of action.
Conclusion
Viral immunotherapy is emerging as a promising treatment modality for malignant glioma therapy. PVS(RIPO) is at the forefront of this innovative treatment as highlighted by the Food and Drug Administration granting Breakthrough Therapy Designation. This aims to expedite the development process based on the successful preliminary results from the ongoing clinical trial. As PVS(RIPO) proceeds through the clinical trial process, its basic mechanisms of translational regulation, cytotoxicity, and immunogenic response continue to be investigated and elucidated. Clarifying these mechanisms will likely lead to further development and enhancement of the clinical application for oncolytic viral immunotherapies.